Recently Posted
-

Will AI have a Positive Effect on Eye Care Specialists?
 Maria Martynova
18.03.202313 min read
Maria Martynova
18.03.202313 min readWill AI improve your practice or it’s another hype topic that will vanish like NFT or VR glasses?
This article examines present AI’s impact on eye care specialists, exploring its promises and challenges. To gain a realistic view, we surveyed eye care specialists on their experiences and expectations of this topic.
Let’s start with what has already been implemented in eye care and the results we can see already.
FDA-cleared AI for OCT Analysis
AI in Eye Care Industry: Current Status
Disease screening: DR, AMD, and rare pathologies & biomarkers
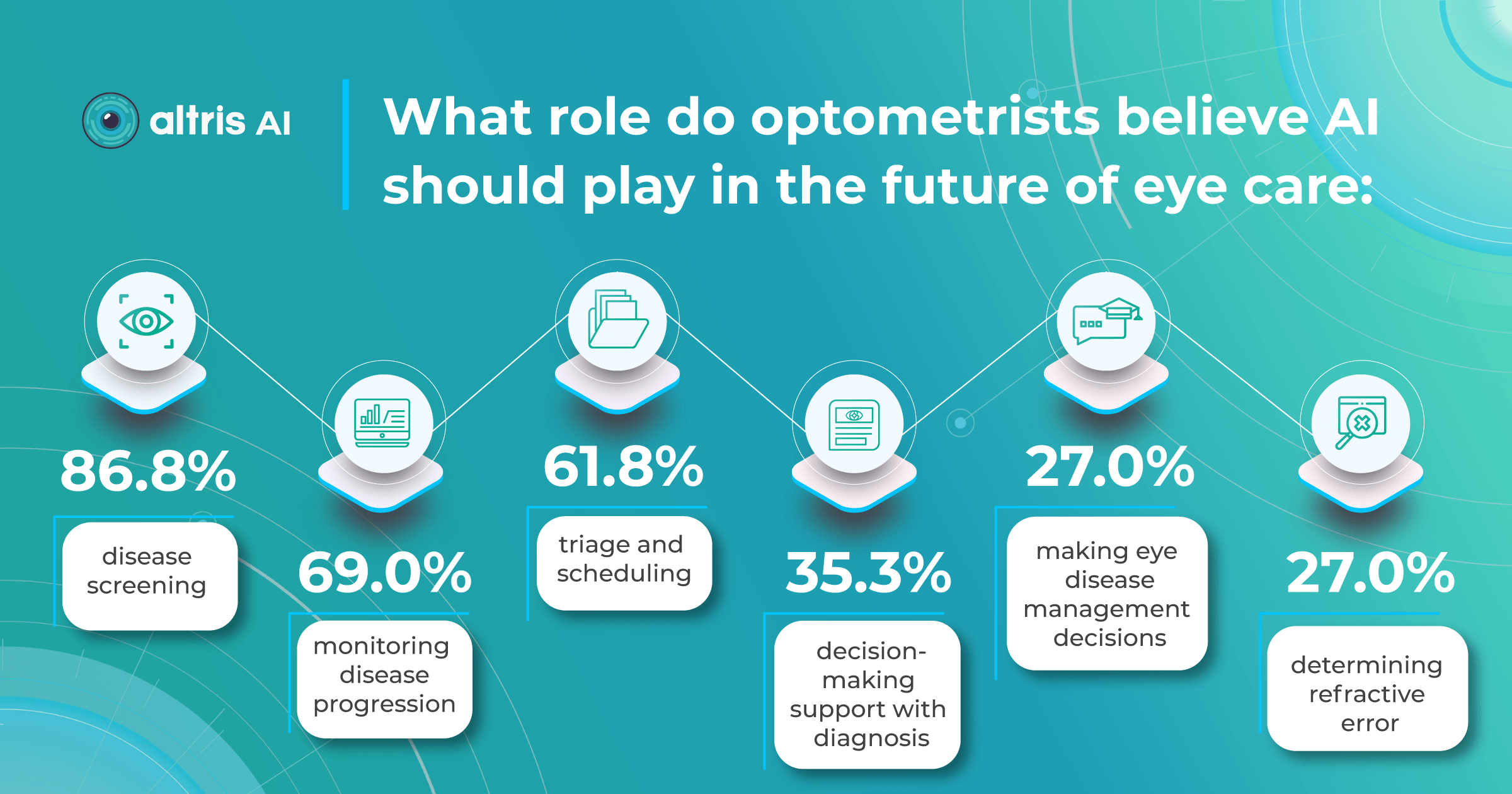
A 2022 study by the University of Illinois showed that eye care specialists mostly see AI helping with disease screening, monitoring, and patient triage tasks. Notably, a significant increase in willingness to incorporate AI in practice has emerged after the COVID-19 pandemic, presumably due to a need for remote consultations.
The growing interest in AI for disease screening and monitoring coincides with the development of sophisticated AI systems. Due to their significant causes of visual impairment, Diabetic Retinopathy and AMD are the primary targets for AI screenings.
With over 422 million people worldwide affected by diabetic retinopathy and an estimated 80 million suffering from age-related macular degeneration, the workload on eye care specialists is immense. Unsurprisingly, most AI-powered screening solutions focus on helping clinicians with these diagnoses.
AI algorithms are trained to recognize DR-related alterations on images: hemorrhages, exudates, and neovascularization. AI also offers significant advancements in Age-related Macular Degeneration screening. Algorithms accurately segment data in OCT scans, helping assess retinal structures and quantify fluids during treatment. Trained models predict disease progression risks and analyze treatment responses.
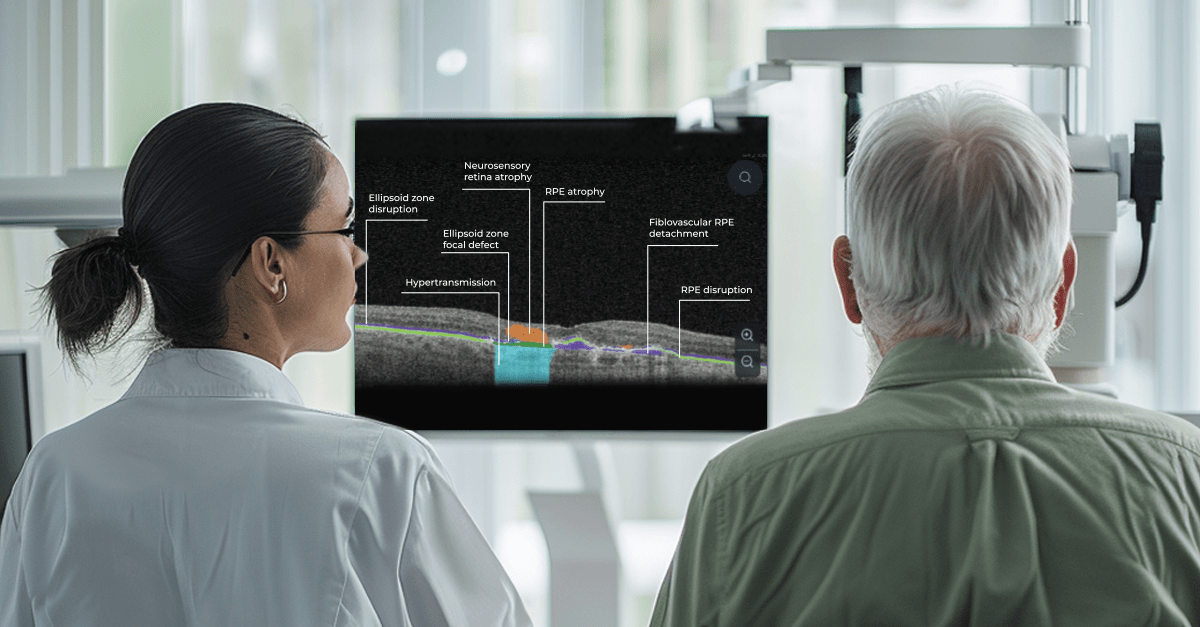
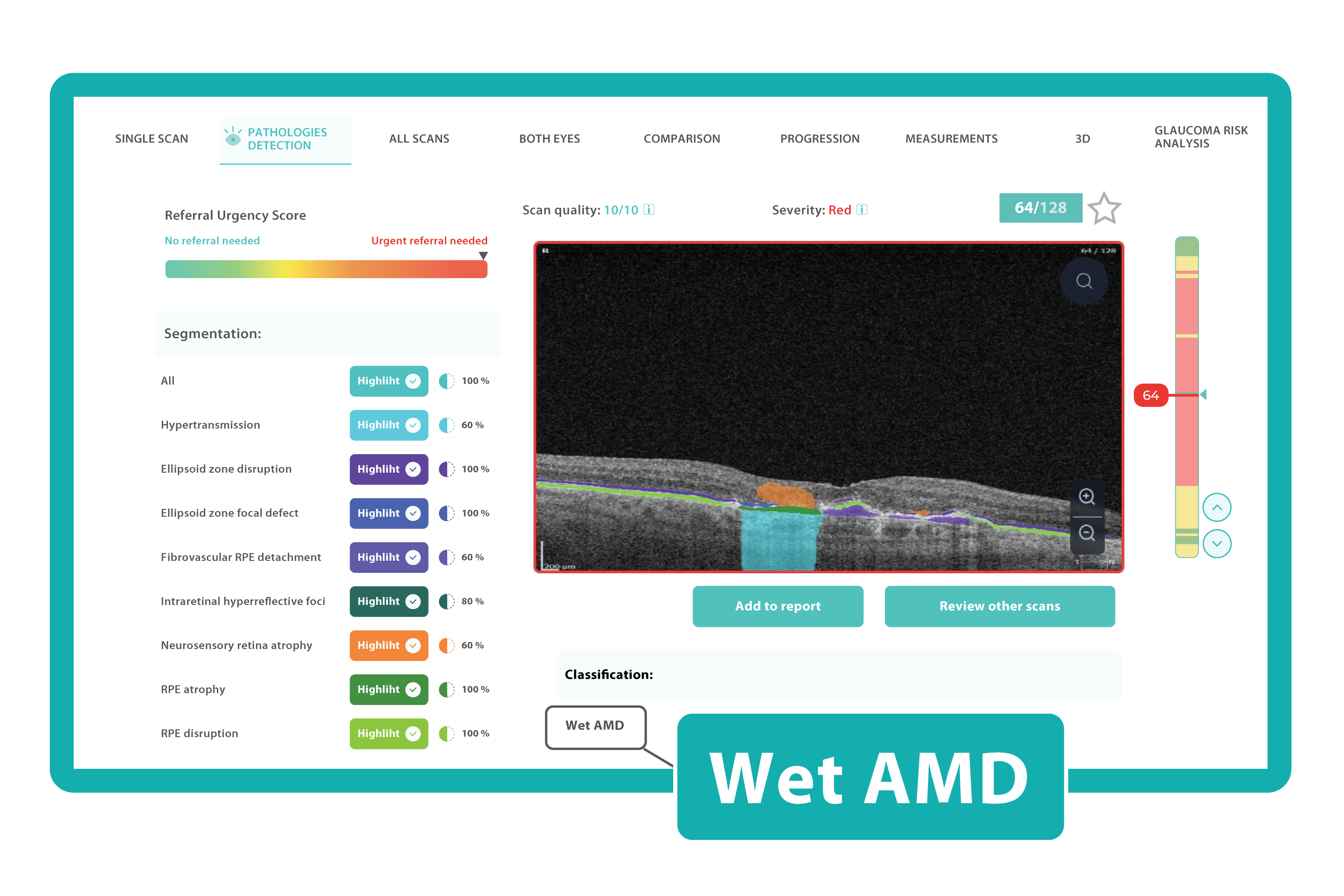 AI in eye care can segment retinal structures to distinguish between normal retina scans and pathology on OCT, detect atrophic changes, and follow all alterations over time. It can even highlight rare inherited retinal dystrophies. For example, Altris AI is trained to recognize Vitelliform dystrophy and Macular telangiectasia type 2.
AI in eye care can segment retinal structures to distinguish between normal retina scans and pathology on OCT, detect atrophic changes, and follow all alterations over time. It can even highlight rare inherited retinal dystrophies. For example, Altris AI is trained to recognize Vitelliform dystrophy and Macular telangiectasia type 2.More Efficient Patient Triage
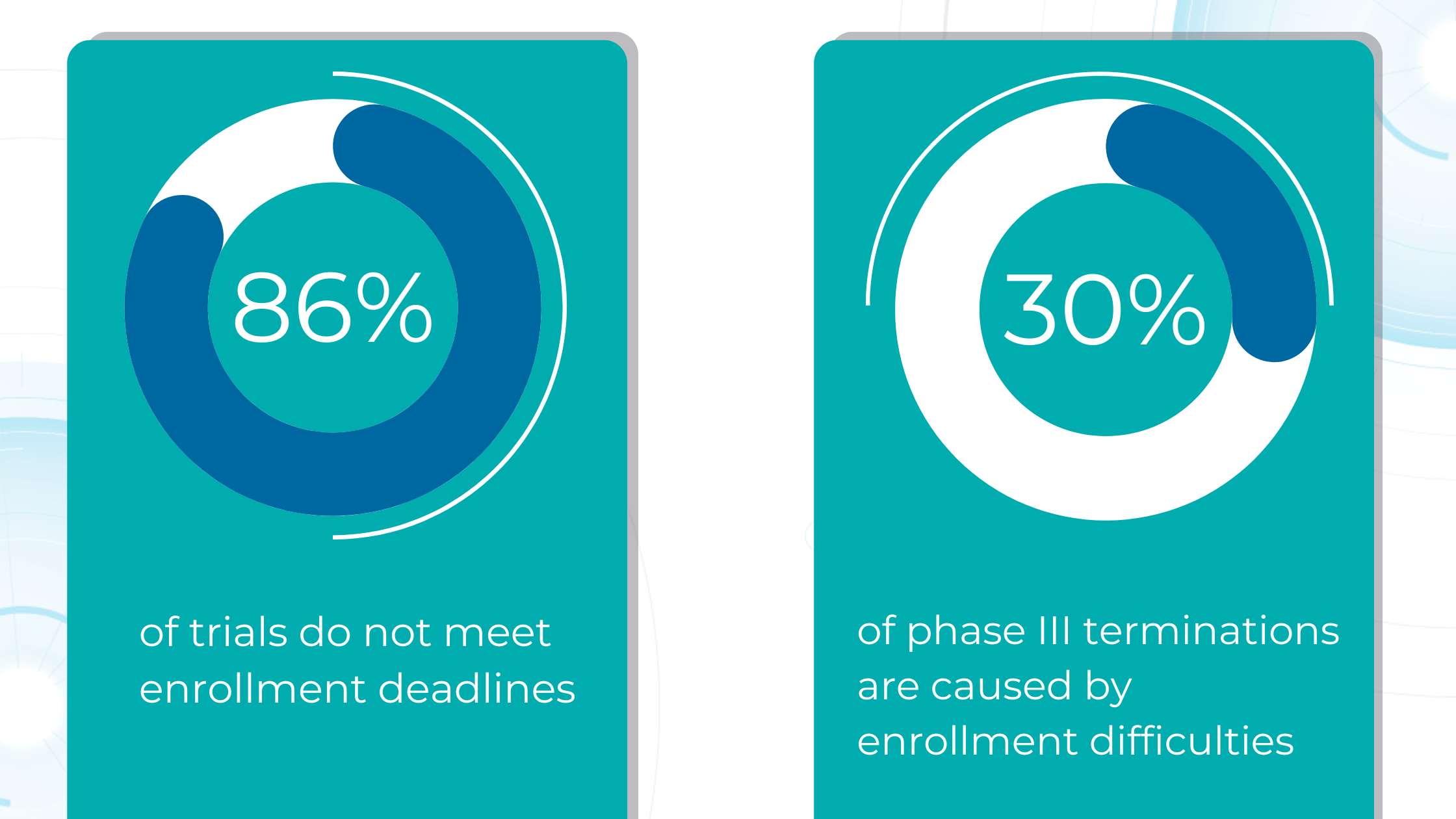
The number of eye scans clinicians are performing is growing at a pace much faster than human experts are able to interpret them. This delays the diagnosis and treatment of sight-threatening diseases, sometimes with devastating results for patients.
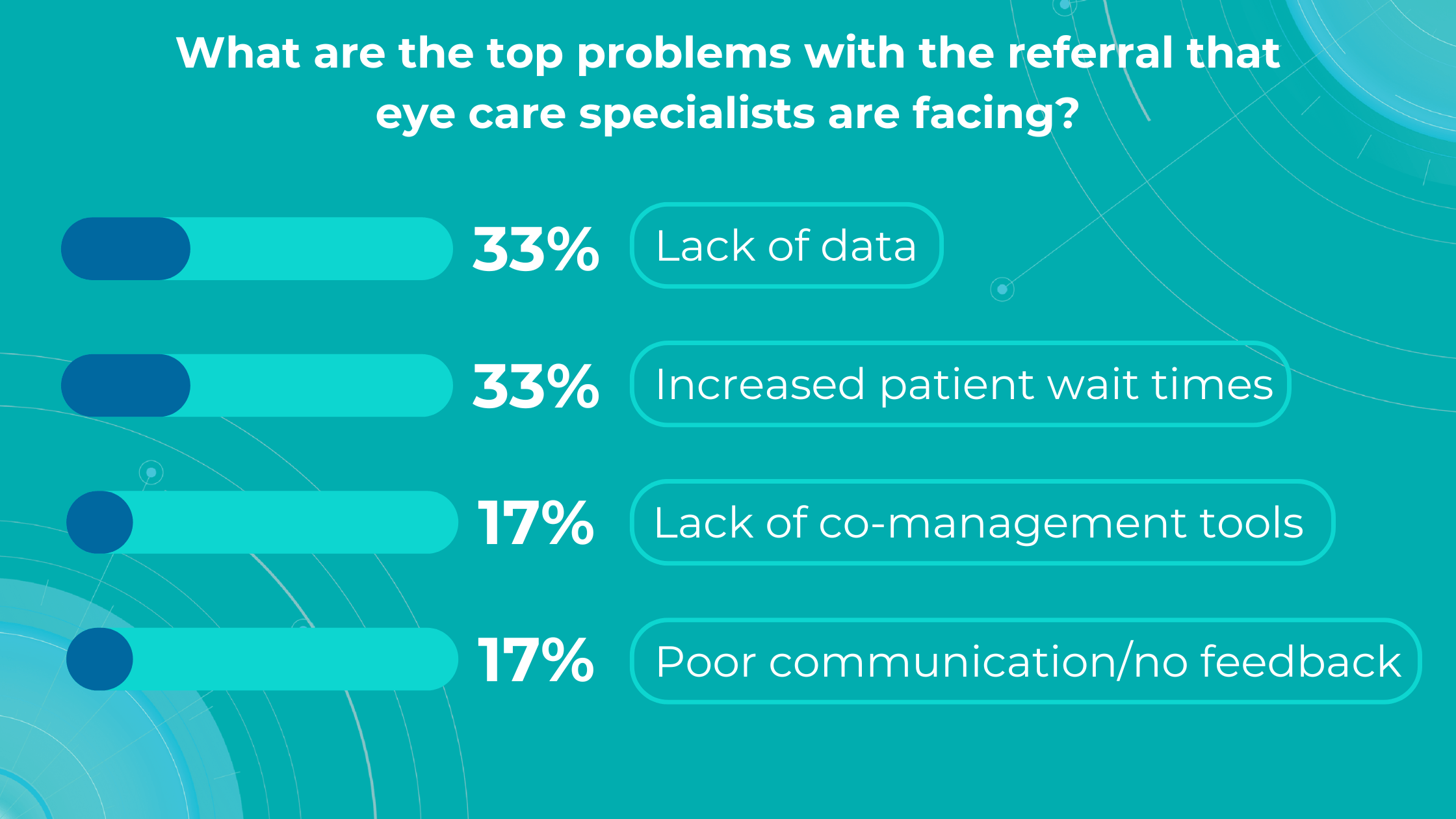
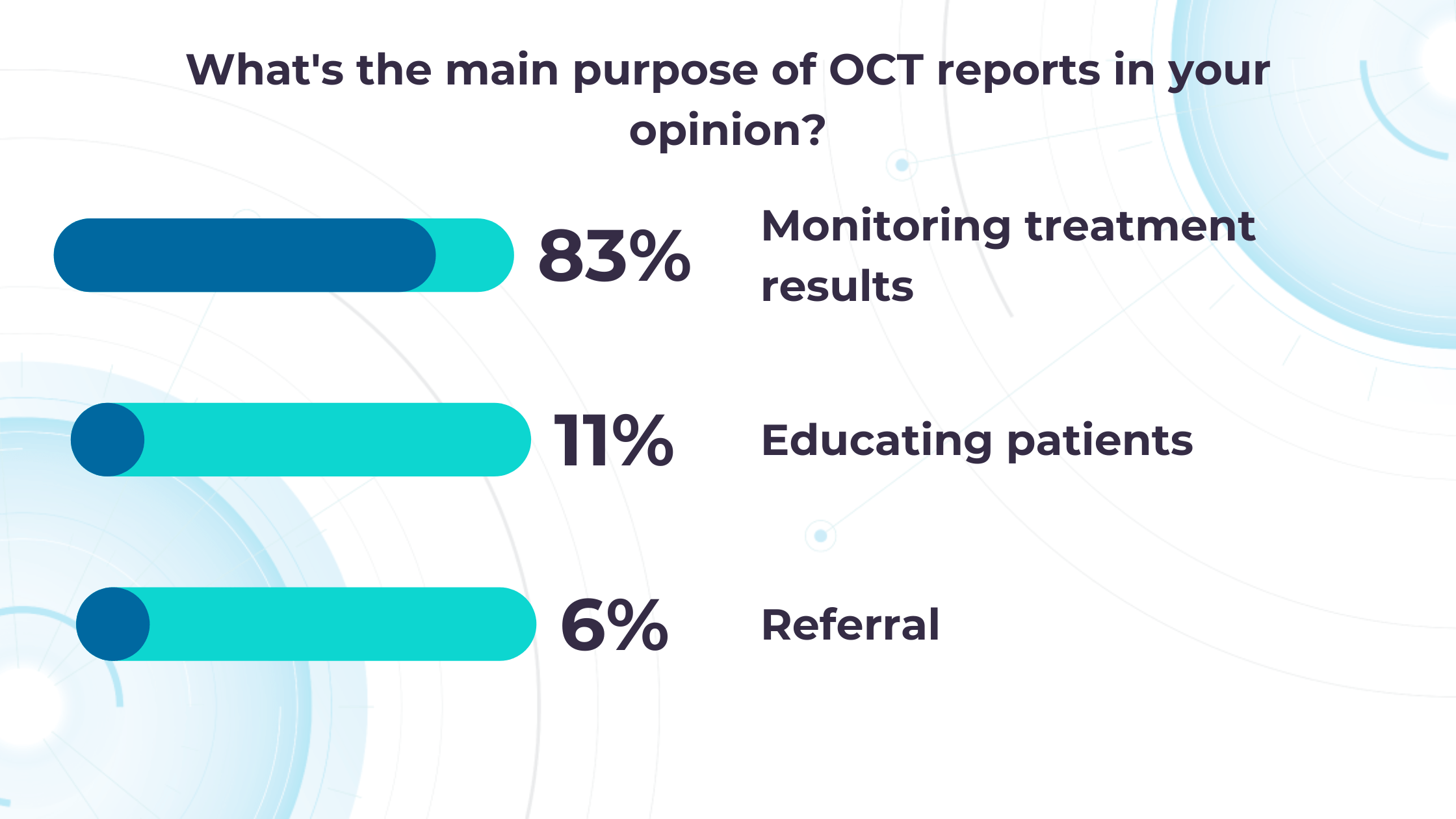
Our recent survey showed that among more than 1000 participating eye care specialists, 40% have more than 10 OCT exams daily. Meanwhile, 35% of eye care specialists have 5-10 OCT daily examinations. Unfortunately, more patients per day mean an increased risk that specialists may miss some minor, rare, or early conditions.

AI systems can quickly triage scans based on severity. Prioritized urgent cases can be flagged for immediate attention. Healthy patients can be monitored without urgency.
This ensures patients with time-sensitive conditions get the care they need, while less urgent cases receive a timely but less immediate review.
Optometrists can use AI systems to specify the need to refer patients based on eye image analysis.
Another advantage of AI used as a “copilot” is its continuous improvement. Providers that create such systems usually integrate new data and research findings into algorithms, resulting in an ever-evolving resource for eye care specialists.
In other words, the accuracy of the patients’ triage will get better and better with the data.
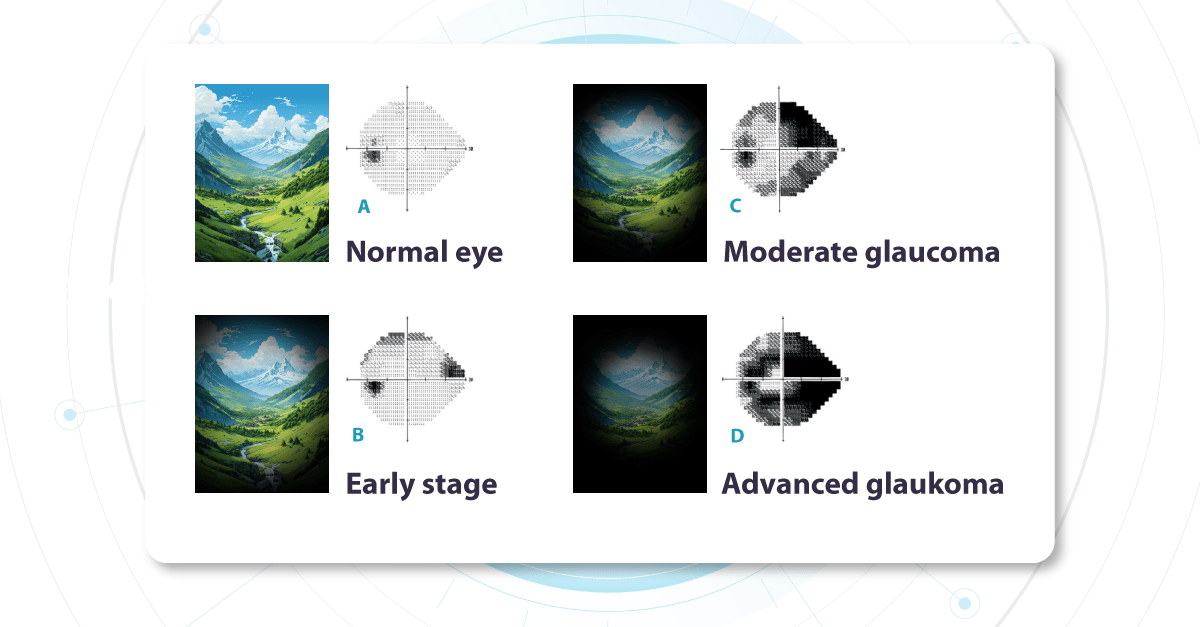
Early Glaucoma Detection
Glaucoma is a leading cause of vision-related morbidity worldwide. Although blindness is the most feared outcome, even mild visual field loss may harm the quality of life.
In a way, glaucoma is one of the most challenging eye diseases that specialists must treat; with most eye problems, the patient comes when something is wrong. Glaucoma, however, has no symptoms until it is advanced, and the damage can not be reversed.
One common reason glaucoma is not diagnosed early is the inability to recognize glaucomatous optic disc and RNFL damage. Ophthalmologists often rely primarily on intraocular pressure and visual fields and not on the appearance of the optic disc.
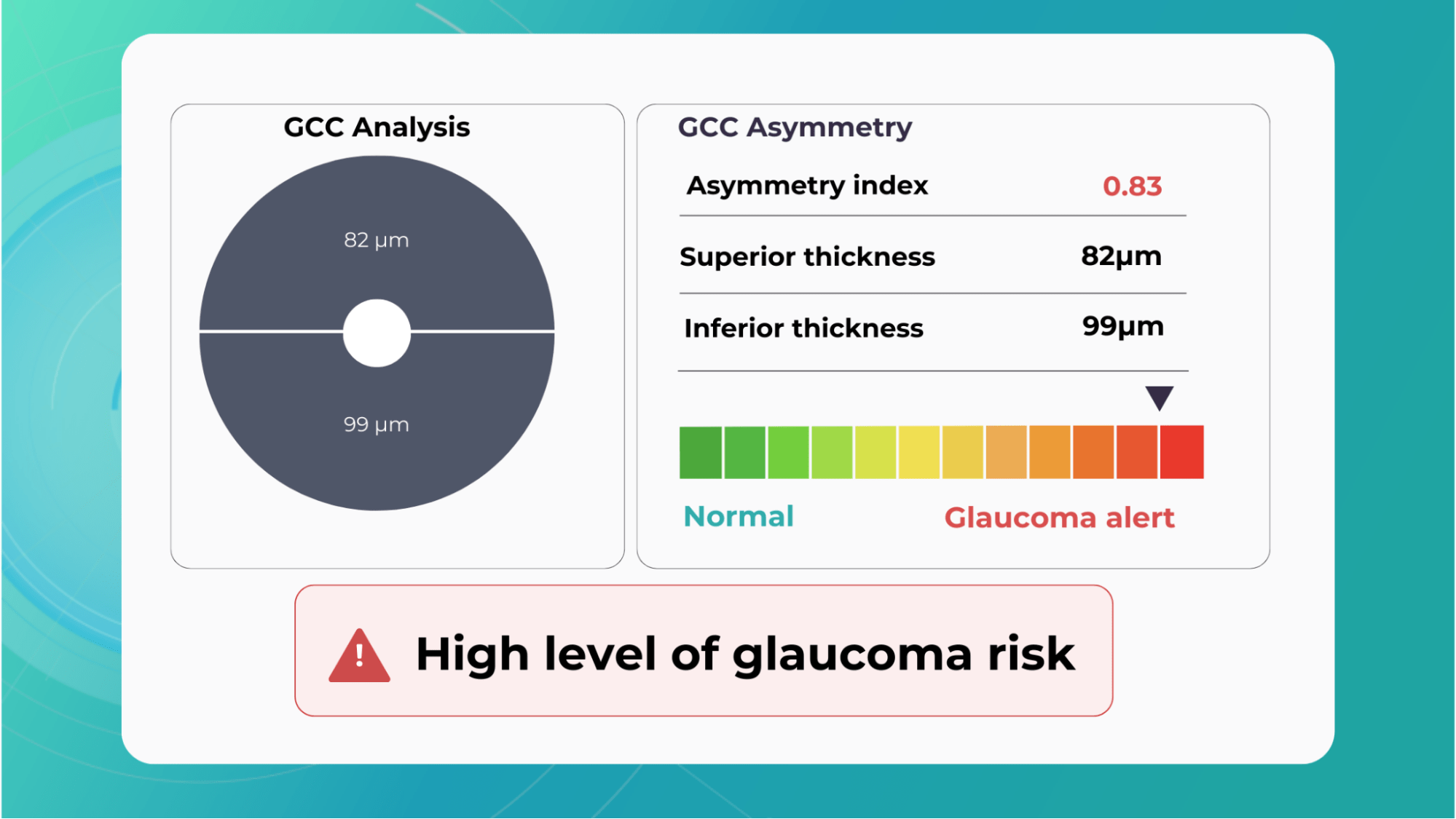
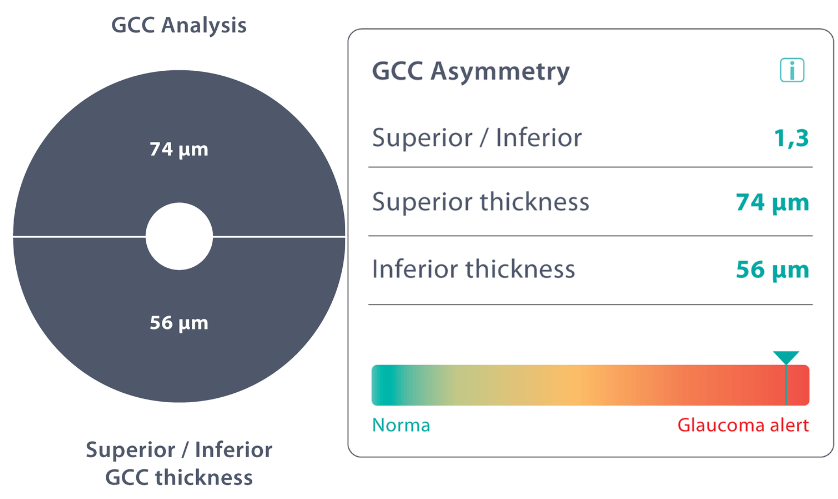
Combining optical coherence tomography imaging and artificial intelligence, Altris AI offers a solution to the problem. The platform performs Ganglion Cell Complex asymmetry analysis on OCT scan that categorizes the risk of developing glaucoma. Glaucoma Early Risk Assessment Module can help decrease the number of false-positive referrals and increase the standard of care by supporting early diagnosis to improve patients’ prognosis.
Better Education for Patients
Eye care specialists don’t always have time to explain to patients what is going on with their eye health.
Artificial intelligence can easily perform this task. AI systems will also enhance eye care education, offering innovative and immersive learning experiences: with the help of color-coding, user-friendly reports, and chat bots.
AI-generated OCT reports can propel patient education and engagement. By translating complex medical data into clear, visual formats, AI can help understand patients’ diagnoses, significantly improving treatment adherence and fostering greater patient loyalty.
For example, Altris AI employs smart reports with color-coded segmentation of pathologies that are easy for clinicians and their patients to understand.
When patients fully grasp the nature of their eye conditions and track therapy progress, they are far more likely to prioritize annual checkups and actively engage in their care.
Teleoptometry and teleophthalmology
The COVID-19 pandemic has accelerated the adoption of telemedicine, especially in the image-rich field of ophthalmology.
In recent years, many digital home measurement tests have been introduced. These include home-based and smartphone/tablet-based devices, which are cost-effective in specific patient cohorts.
One example is an artificial intelligence-enabled program for monitoring neovascular Age-related Macular Degeneration (nAMD) that uses a home-based OCT device. Patient self-measurements from home have proved to be a valuable adjunct to teleophthalmology. In addition to reducing the need for clinical visits, they serve as a collection of high-quality personal data that can guide targeted management.
Currently, most commercial providers of telemedical services and devices use artificial intelligence. However, these services are not autonomous. AI works simultaneously with so-called “backup” ophthalmologists. If a finding is unknown or unclear to the artificial intelligence, an ophthalmologist reads the image.
Non-medical AI: General Workflow Enhancements
COVID-19 made it crystal clear that healthcare worldwide has a full spectrum of problems, such as staffing shortages, fragmented technologies, and administrative complexities. So, the AI boom three years after the pandemic has come timely and handy.

Intelligent algorithms can solve the mentioned issues. For example, generative AI can enable easier document creation by digesting all types of reports and streamlining them. It can also ease the administrative workload for short-staffed clinicians (the average US nurse spends 25% of their work time on regulatory and administrative activities).
Probabilistic matching of data across different databases, typical for Machine Learning, is another technology that can take a burden off staff about claims and payment administration.
Patient engagement and adherence also can benefit from the technology. Providers and hospitals often use their expertise to develop a plan to improve a patient’s health, but that frequently doesn’t matter as the patient fails to make the behavioural adjustment. AI-based capabilities can personalize and contextualize care, using machine learning for nuanced interventions. It can be messaging alerts and targeted content that provokes actions at needed moments or better-designed ‘choice architecture’ in healthcare apps.
Another side of the coin: AI for OCT limitations
When discussing AI in eye care, it’s essential to recognize that AI is a tool. Like any tool, it is neutral. So, its effectiveness and potential for unintended consequences hinge not only on the quality of its design and the data used to train it but also on the expertise of the healthcare professionals interpreting its output. Here are some of the challenges to keep in mind when working with AI.
AI is fundamentally limited by the datasets used for training. An outsized amount of images can slow training and lead to overfitting, while a lack of demographic diversity compromises accuracy.
One challenge facing AI implementation in medicine is the interdisciplinary gap between technological development and clinical expertise. These fields are developing separately and usually do not intersect. Therefore, cross-collaboration can suffer because tech experts may not understand medical needs, and clinicians may not have the technical knowledge to guide AI development effectively.
So, a successful AI solution requires bridging this breach to ensure AI solutions are grounded in medical realities and address the specific needs of clinicians (Clinical & Experimental Ophthalmology, 2019).
The commercialization of AI will also pose future issues. Trained models will likely be sold with and for implementation with certain medical technologies. Additionally, if AI does improve medical care, it will be essential to pass those improvements on to those who cannot afford them.
Overreliance on the technology can also be a problem.

AI is a tool, like any other equipment in the clinical environment. Decision-making is always on the side of an eye care practitioner who has to take into account many additional data: clinical history, other lab results, and concomitant diseases in order to make a final diagnosis.
And, of course, there are ethical dilemmas. Many practical problems can be solved relatively easily – secure storage, anonymization, and data encryption to protect patient privacy. However, some of them need a whole new field of law. The regulations surrounding who holds responsibility in case of a misdiagnosis by AI is still a significant question mark. Since most current AI algorithms diagnose not so many diseases, there is room for error by omission, and a correct AI diagnosis is not a comprehensive clinical workup.
Summing up

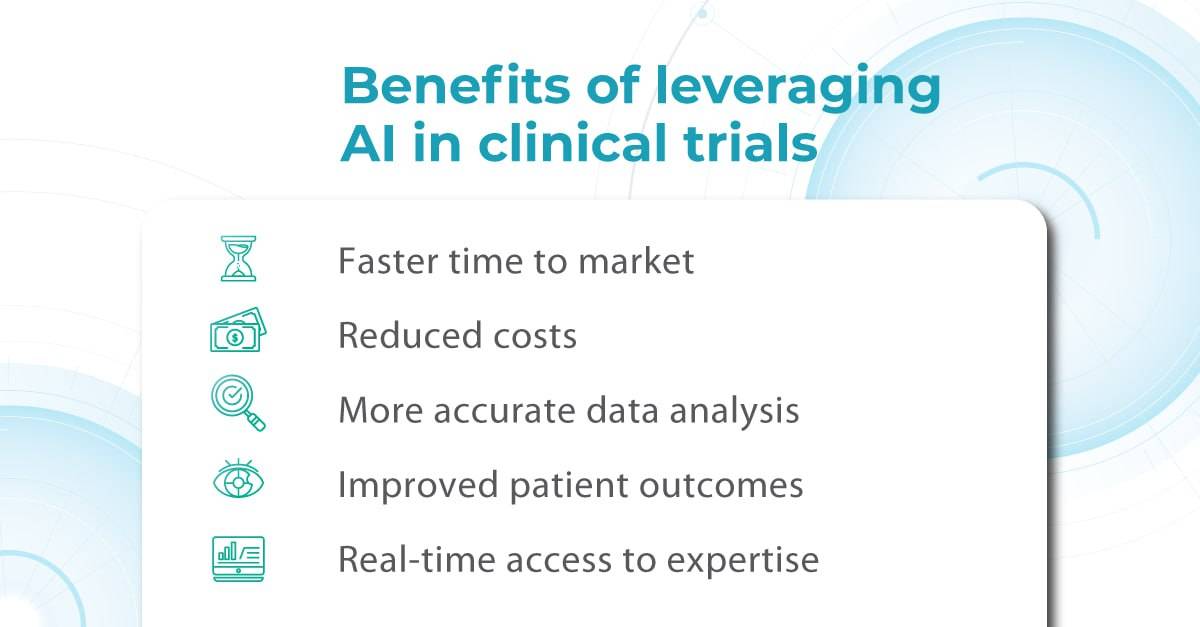
While AI in eye care isn’t without limitations and ethical considerations, its revolutionizing potential is hardly deniable. It already has proven itself working with disease screening, monitoring, and triaging, saving specialists time and improving patient outcomes. AI offers a “second opinion” for complex cases and expands access through telemedicine.

FDA-cleared AI for OCT Analysis
Yet, despite all its promises, the implementation of AI in practice should be seen as a new tool and technique, like the invention of the ophthalmoscope, IOL, OCT, and fundus camera. Optometrists and ophthalmologists will need to combine the best of their clinical skills and AI tools for best practices. Being an innovative tool does not make AI a magic wand, fortunately or not.
-

Technologies in Optometry: Clare and Illingwort & Altris AI
 Altris Team
3 min.3 min.
Altris Team
3 min.3 min.The Client: Clare and Illingworth, renowned leaders in the field of optometry located in the UK.
The problem: The need to speed up the process of OCT interpretation and unburden the optometry team.
The Solution: Clare and Illingworth have embraced cutting-edge technology to enhance their Optical Coherence Tomography (OCT) analysis workflow. The introduction of Altris AI at this optometry center marks a significant milestone in their commitment to providing high-quality services to patients.
According to one of the owners of the optometry center, Richard, “We are adding a new OCT to one of our practices and will benefit from some extra support with AI to speed up the interpretation of results and assist the busy Optometry team.”
Altris AI, a leading provider of artificial intelligence solutions for healthcare, specializes in developing algorithms and software applications that augment medical imaging analysis. The integration of Altris AI into the British Optometry Center’s OCT workflow brings forth a host of advantages, revolutionizing the way eye conditions are diagnosed and managed.

FDA-cleared AI for OCT Analysis
Try it yourself in our Demo Account or get a Brochure
Technologies in Optometry and Ophthalmology: How AI Helps
One of the key benefits of Altris AI is its ability to automate and expedite the analysis of OCT scans. Traditionally, optometrists spent considerable time manually reviewing and interpreting OCT images.
FDA-cleared Altris AI is created to make the OCT workflow more effective
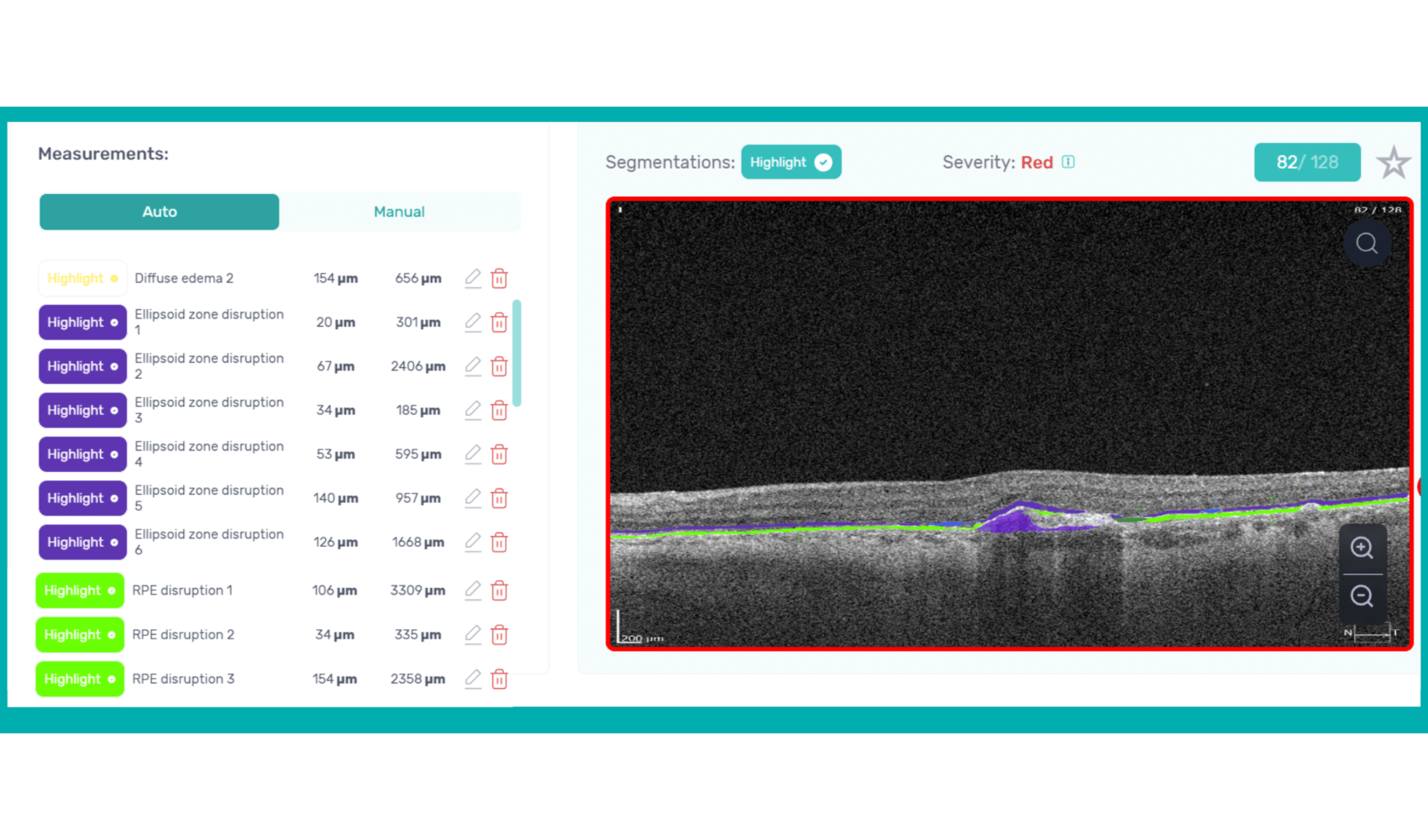
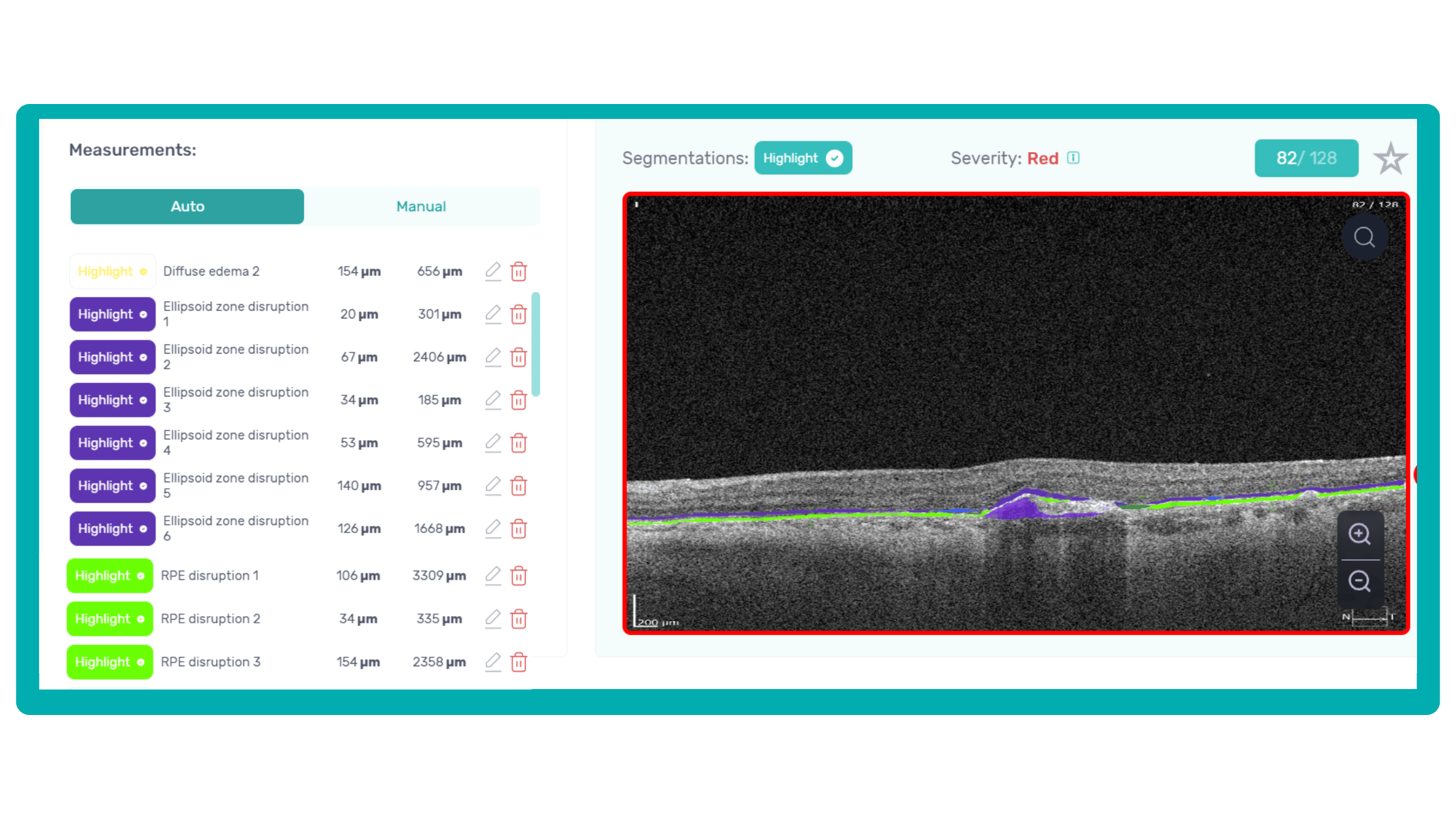
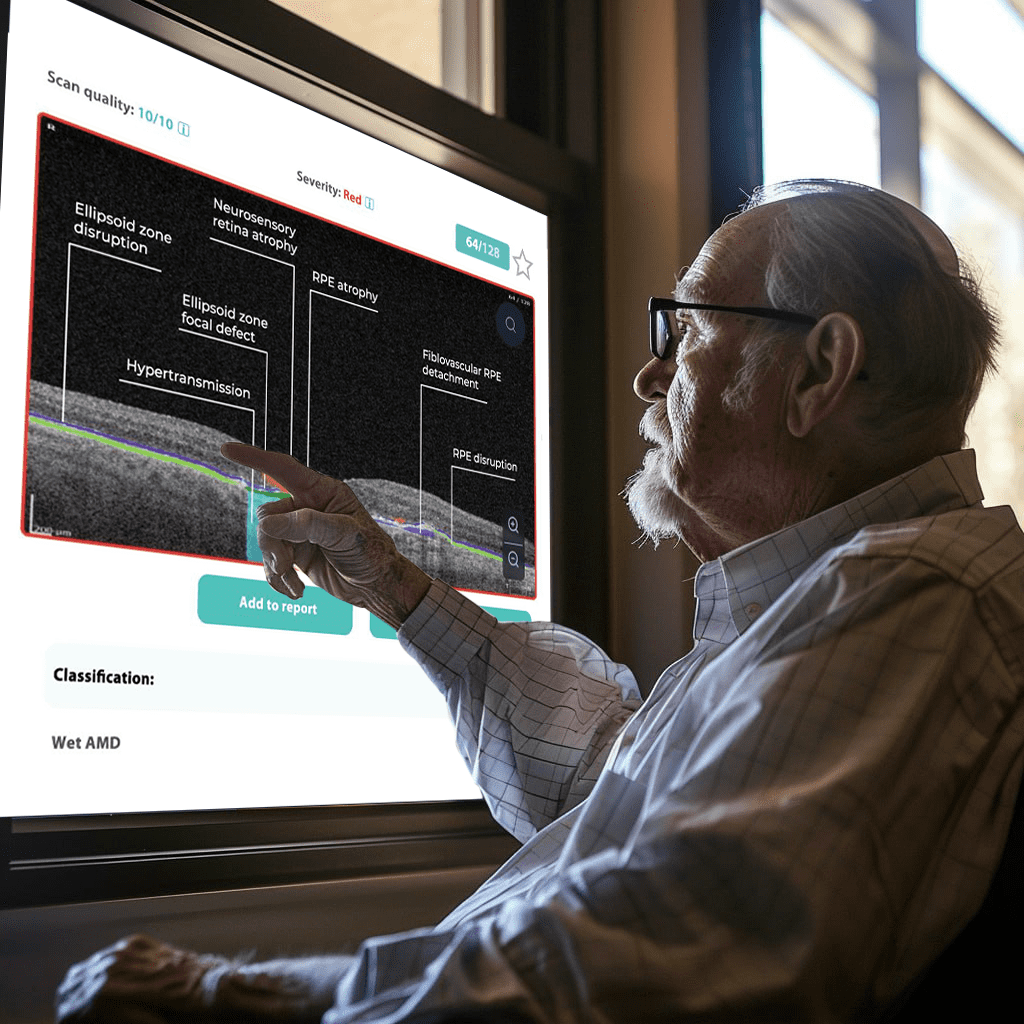
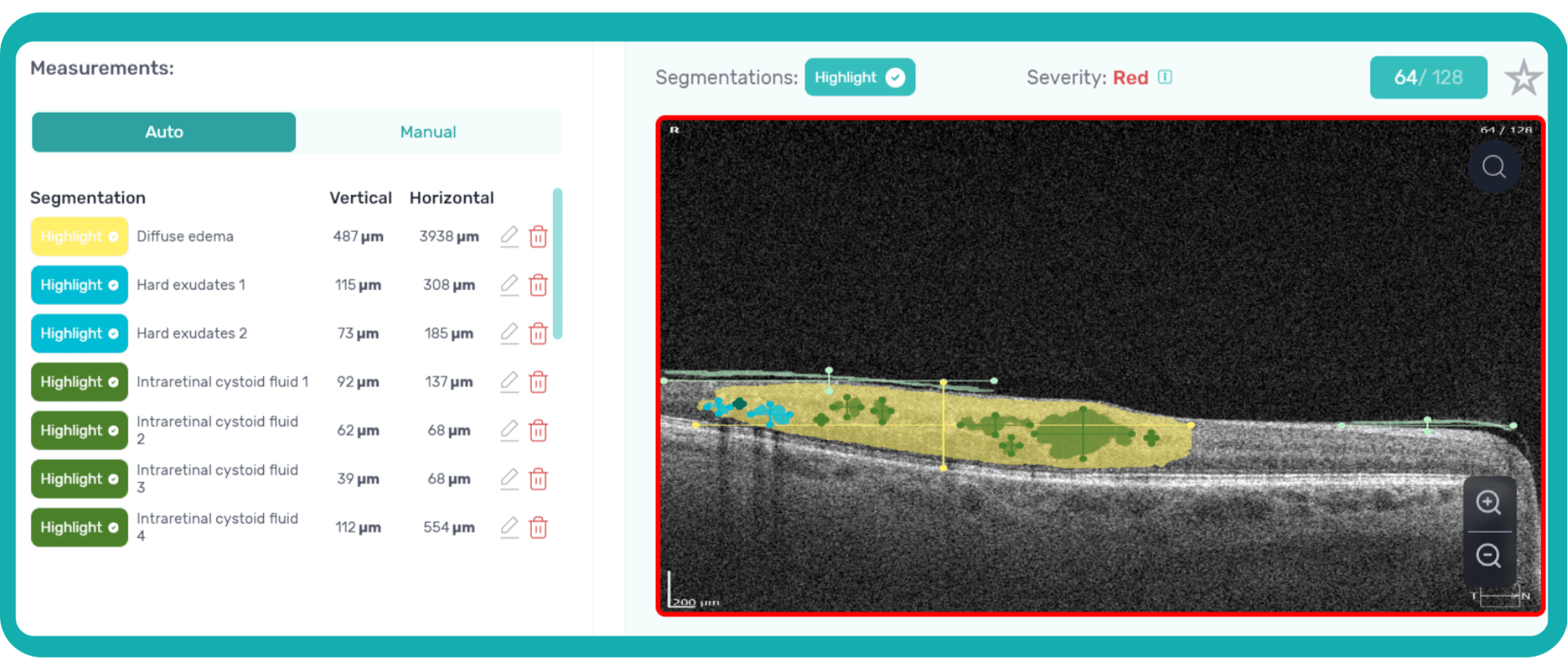
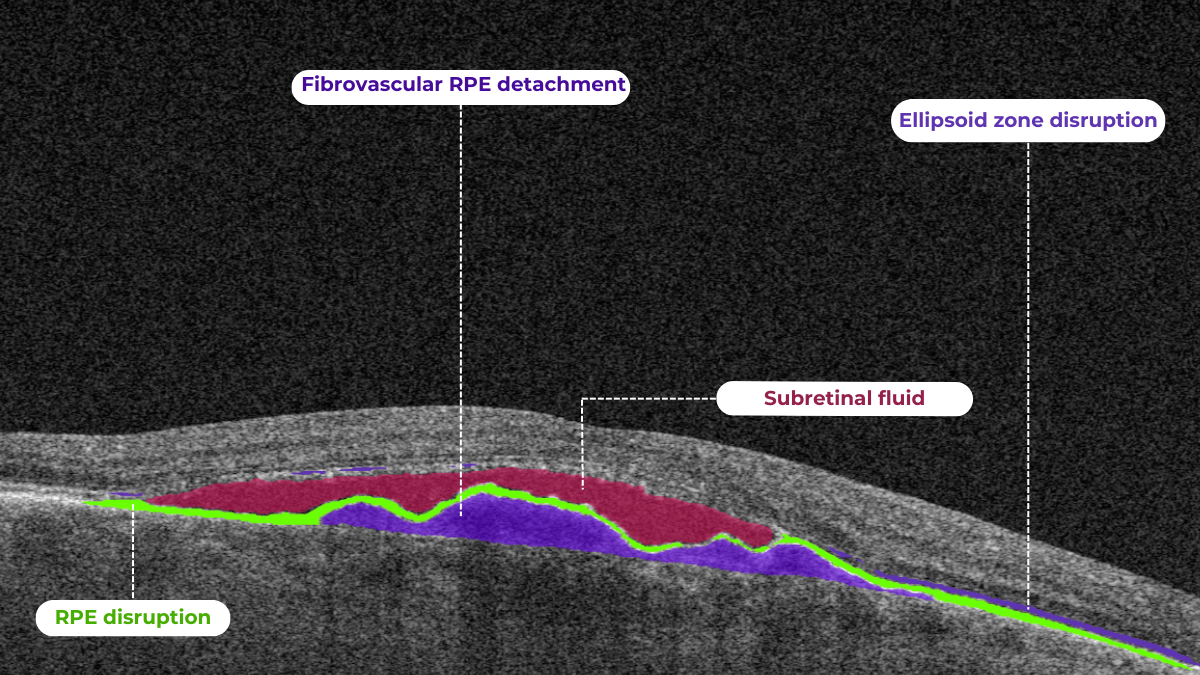
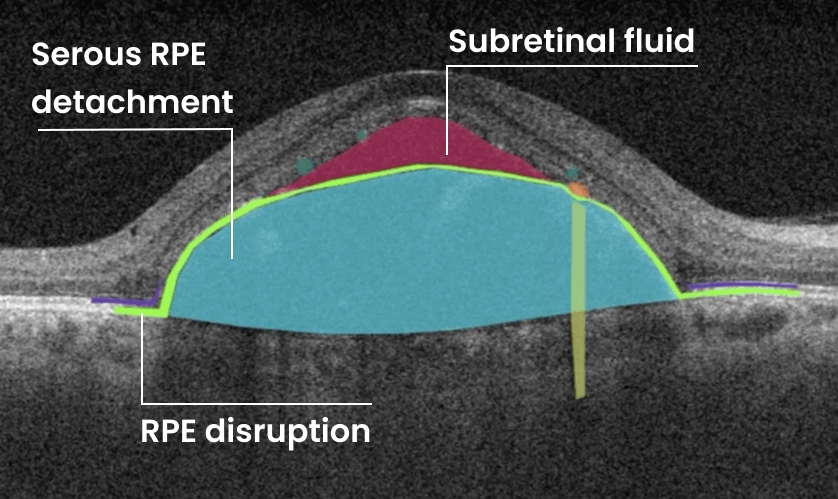
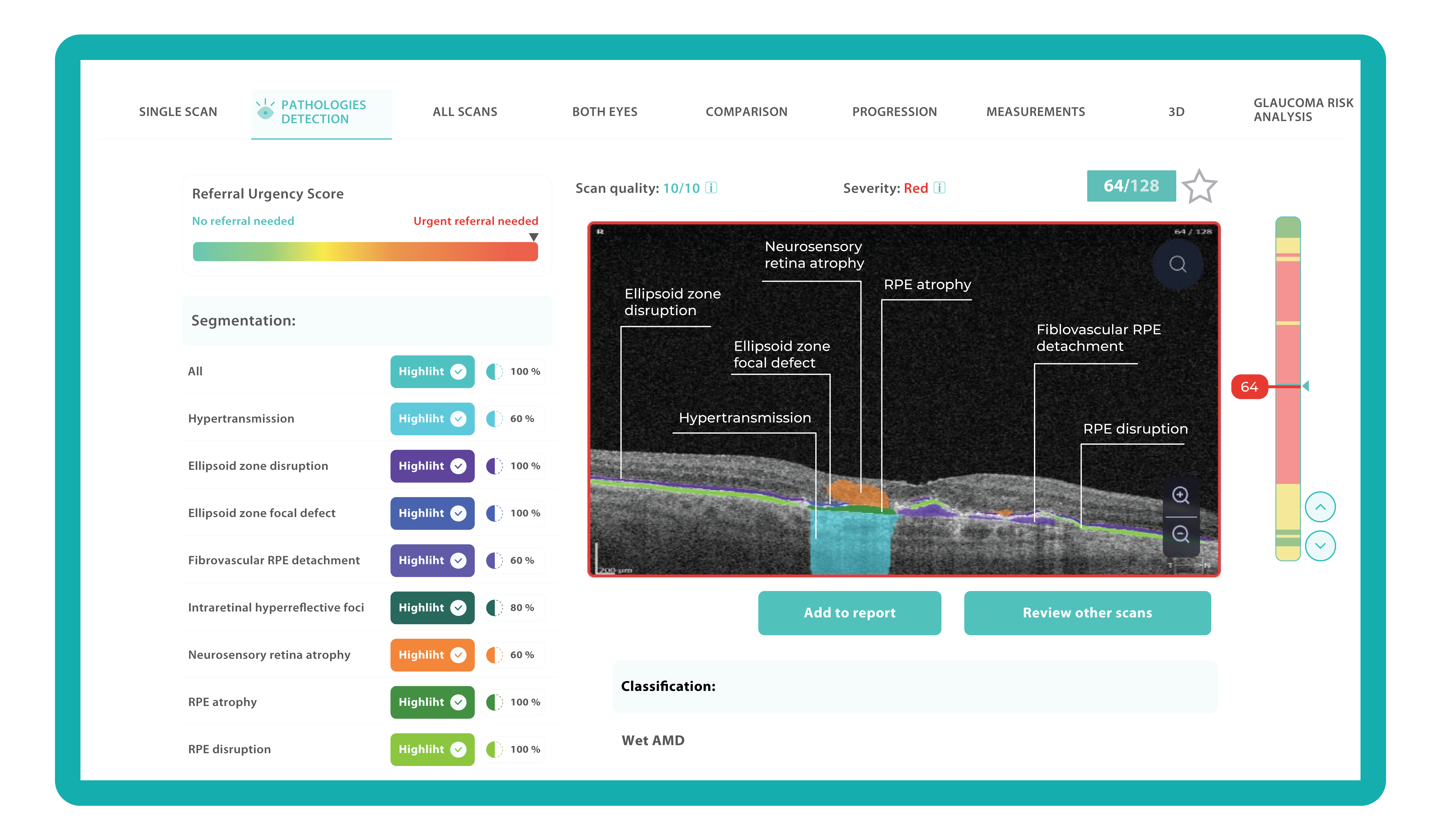
How does it work? Altris AI serves as a copilot, analyzing OCT scans in parallel to the eye care specialist. For instance, on this OCT scan, Altris AI detects Diffuse Edema, Floaters, Intraretinal Hyperreflective Foci, Posterior Hyaloid Membrane Detachment, RPE disruption, Shadowing, Hard Exudates, Intraretinal Cystoid Fluid.
- The classification in this case would be Diabetic Retinopathy.
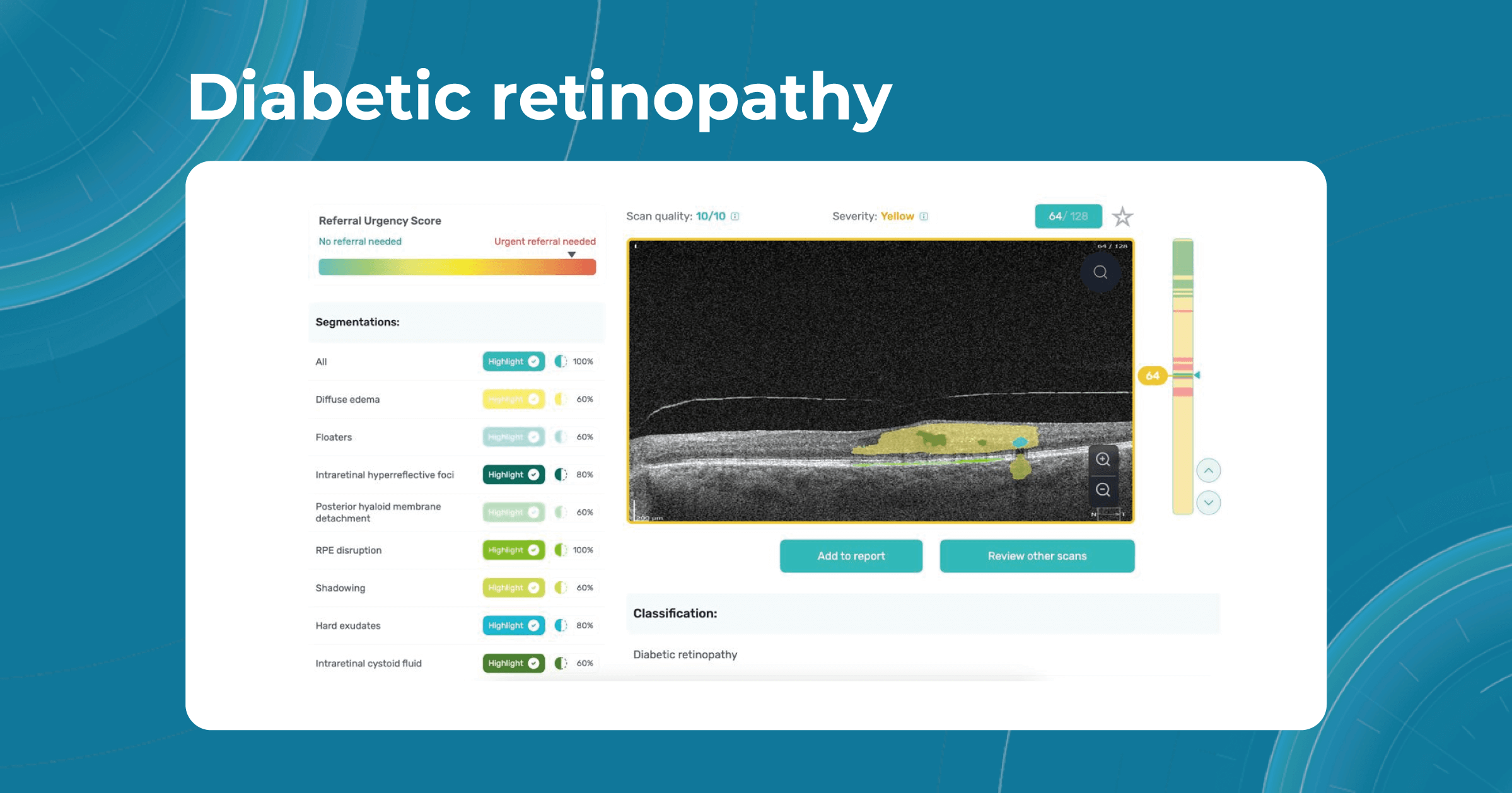
With Altris AI, the process becomes significantly faster and more efficient. The AI algorithms can quickly analyze intricate details within the scans, providing clinicians with accurate and timely insights into the patient’s eye health.
Moreover, the use of Altris AI contributes to increased diagnostic accuracy. The algorithms are trained on vast datasets, learning to recognize subtle patterns and anomalies that may escape the human eye.
Thus, Altris AI recognizes 70+ retina pathologies and biomarkers, including DME, DR, GA, AMD, etc.

FDA-cleared AI for OCT Analysis
Try it yourself in our Demo Account or get a Brochure
Technologies in Optometry are paving the way to a new future where eye care specialists and AI will work together for better patient outcomes. AI will never be able to substitute eye care specialists because the final diagnosis must include clinical history, results of lab tests, and other diagnostic methods.
-

OCT Layers of Retina
 Maria Martynova
5 min.5 min.
Maria Martynova
5 min.5 min.OCT Layers of retina: modern approach to segmentation
The knowledge about macular retinal layer thicknesses and volume is an important diagnostic tool for any eye care professional today. The information about the macular retinal layers often correlates with the evaluation of severity in many pathologies.
Manual segmentation is extremely time-consuming and prone to numerous errors, which is why OCT equipment manufacturers use automatic macular retinal layer thickness segmentation.

Test FDA-cleared AI for OCT analysis
Yet, retina layer segmentation in different OCT equipment manufacturers as well as in different OCT models varies significantly. It is sometimes difficult even for an experienced ECP to find the correlations and track the pathology dynamics. The normative bases refer only to the thickness of the entire retina, they are not related to segmentation. However, if the segmentation is performed incorrectly by the machine, it will lead to an incorrect calculation of the thickness of the retina or its layers, and then the assessment will be incorrect.
At Altris AI we aim to visualize retina layers for a more accurate understanding of pathological process localization. Such retina layers segmentation allows for defining the localization of the pathological process and tracing in dynamics the spread of the pathological process or the aftermath in the retina structure after its completion.
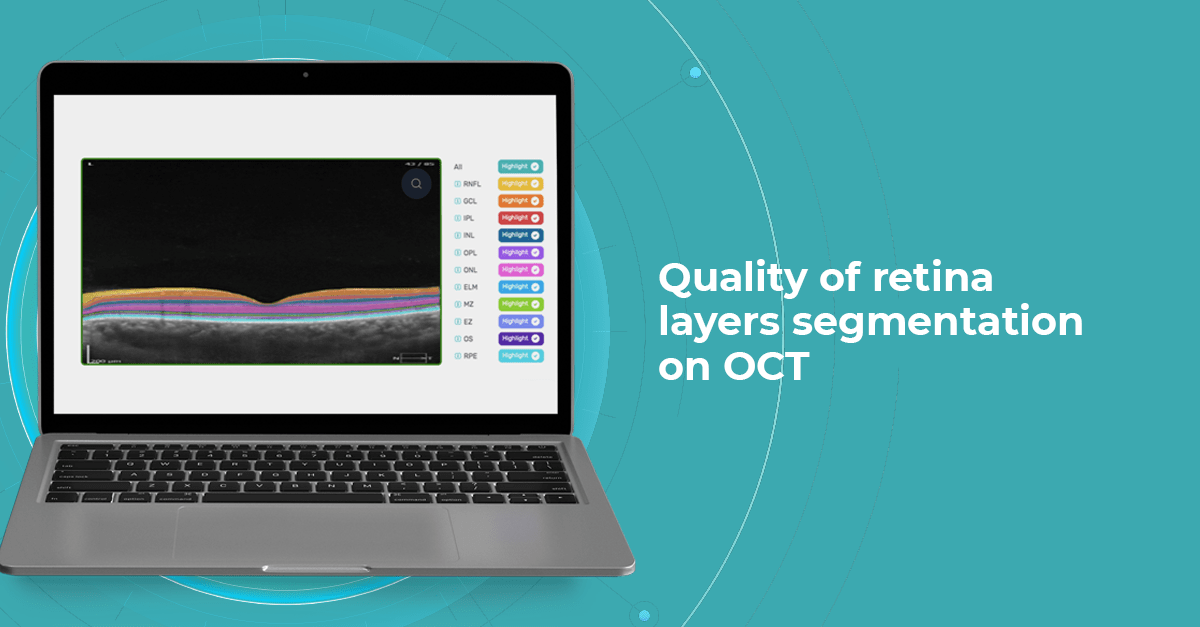
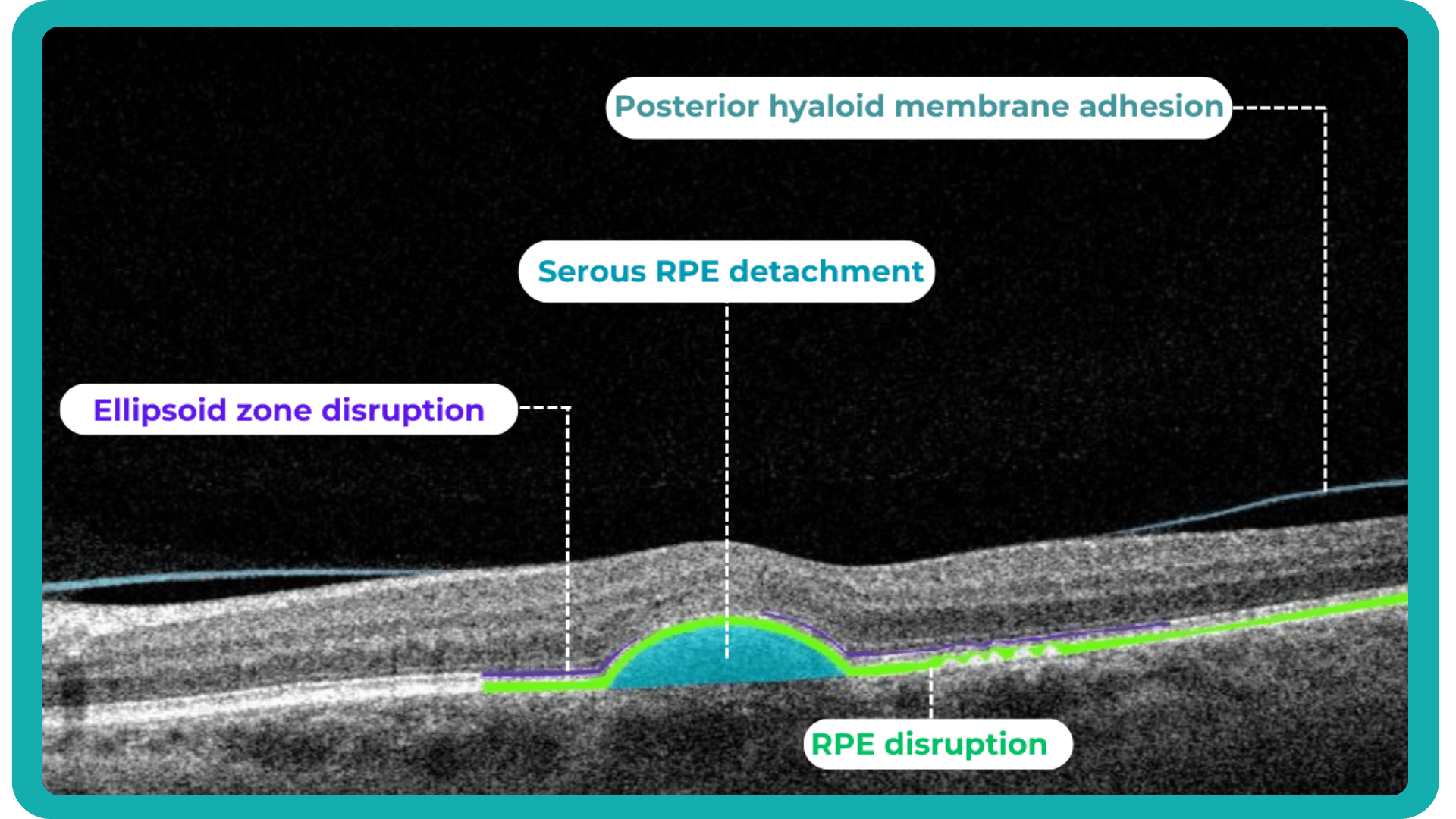
For instance, the EZ layer is important in terms of vision loss forecasting.
OCT Manufacturers & Retina Layers Analysis
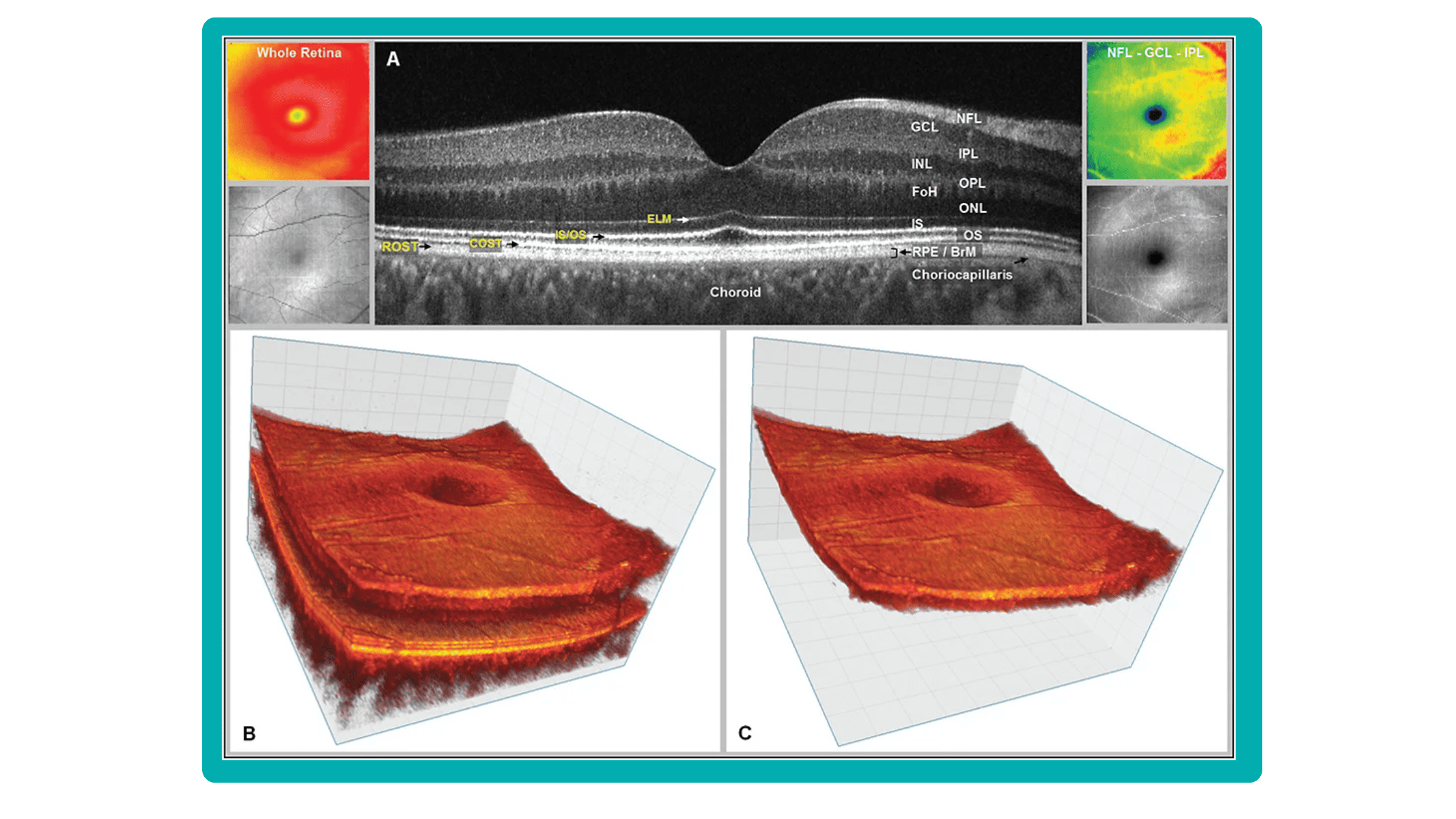
From 2010 most eye care specialists have used the same OCT International Nomenclature for Optical Coherence Tomography. OCT equipment manufacturers rely on this nomenclature for retina layer thickness calculation and most ophthalmologists use it as well.
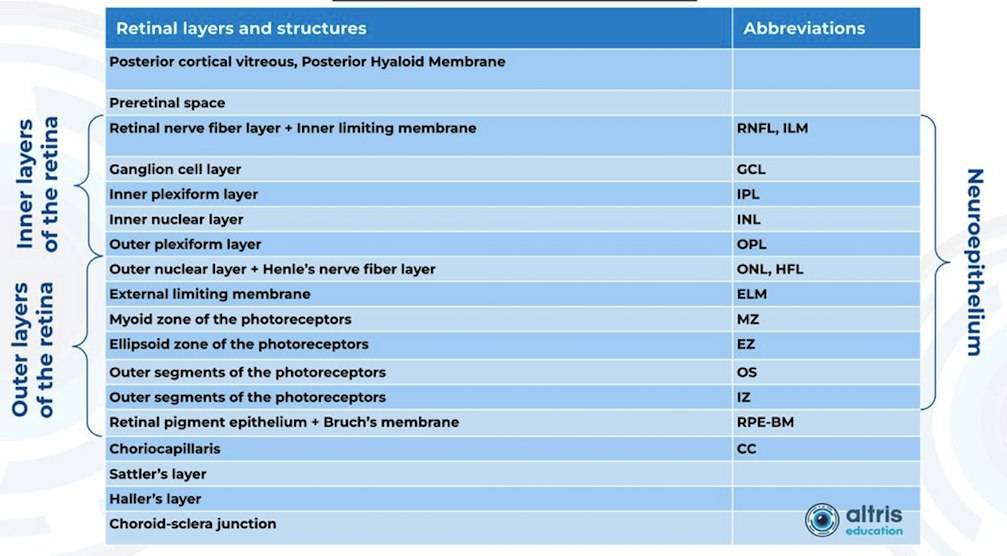
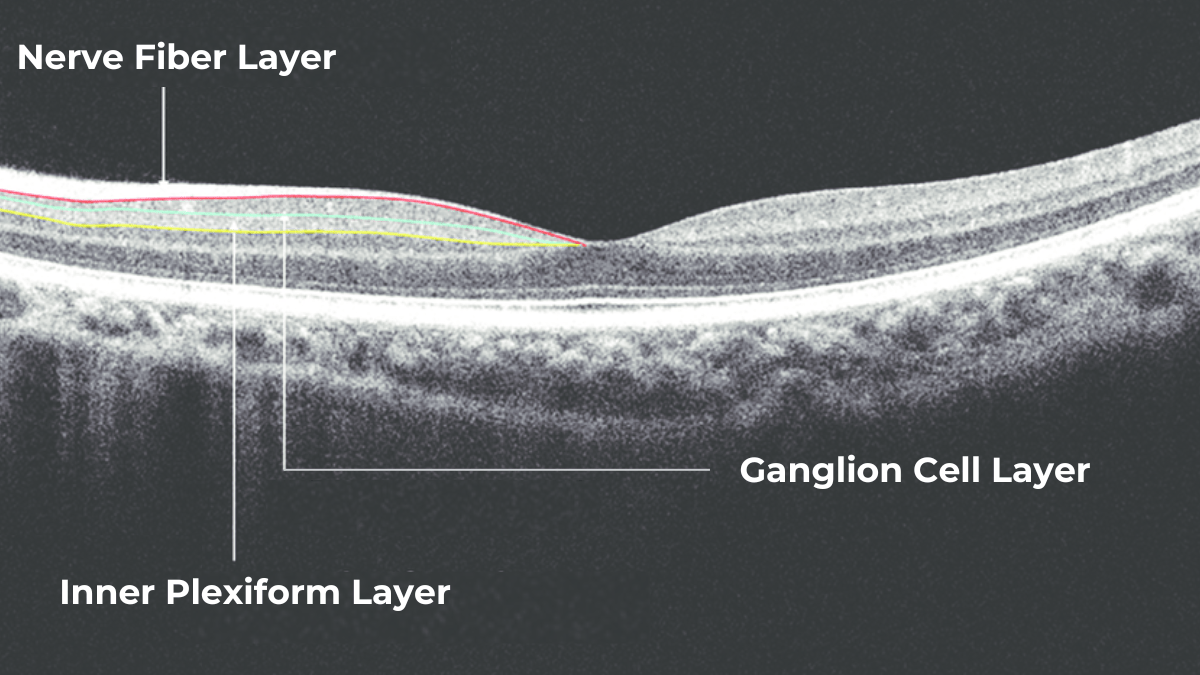
Taking into account retina structure, some layers can be united into complexes. For instance, the ganglion complex includes RNFL, ganglion cell layer & OPL.
Let’s take a look at various OCT equipment manufacturers and the way they perform retina layer segmentation analysis.
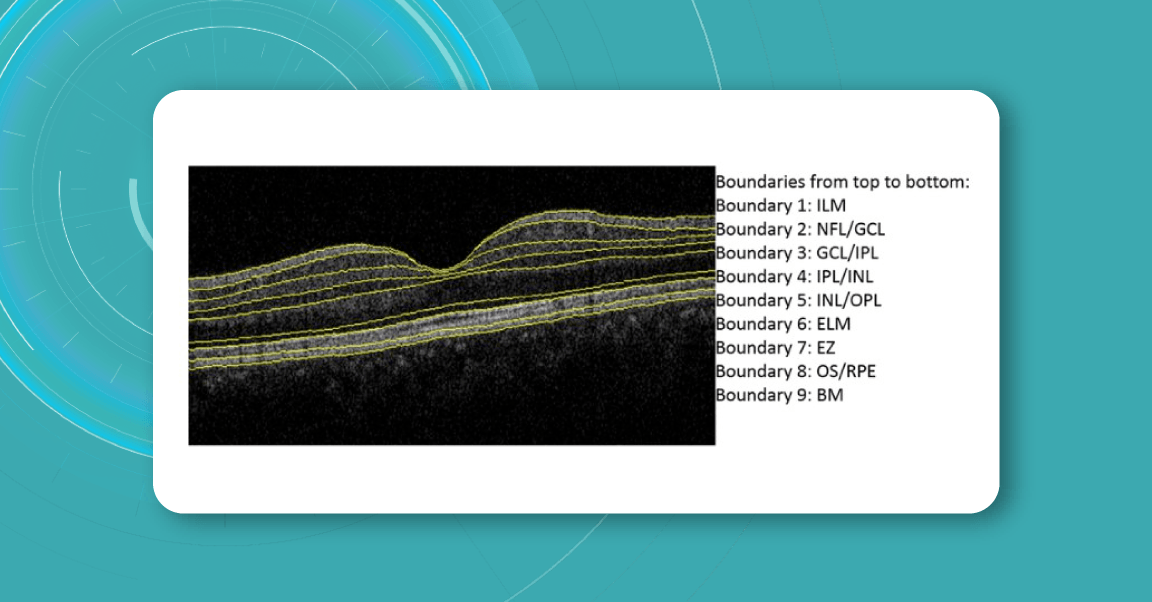
For instance, here is how Topcon Advanced Boundary Segmentation (TABSTM) automated segmentation differentiates between nine intraretinal boundaries:
- ILM
- NFL/GCL,
- GCL/IPL,
- IPL/INL,
- INL/OPL,
- ELM
- EZ
- OS/RPE
- BM
Zeiss CIRRUS uses two approaches to retina layer segmentation.
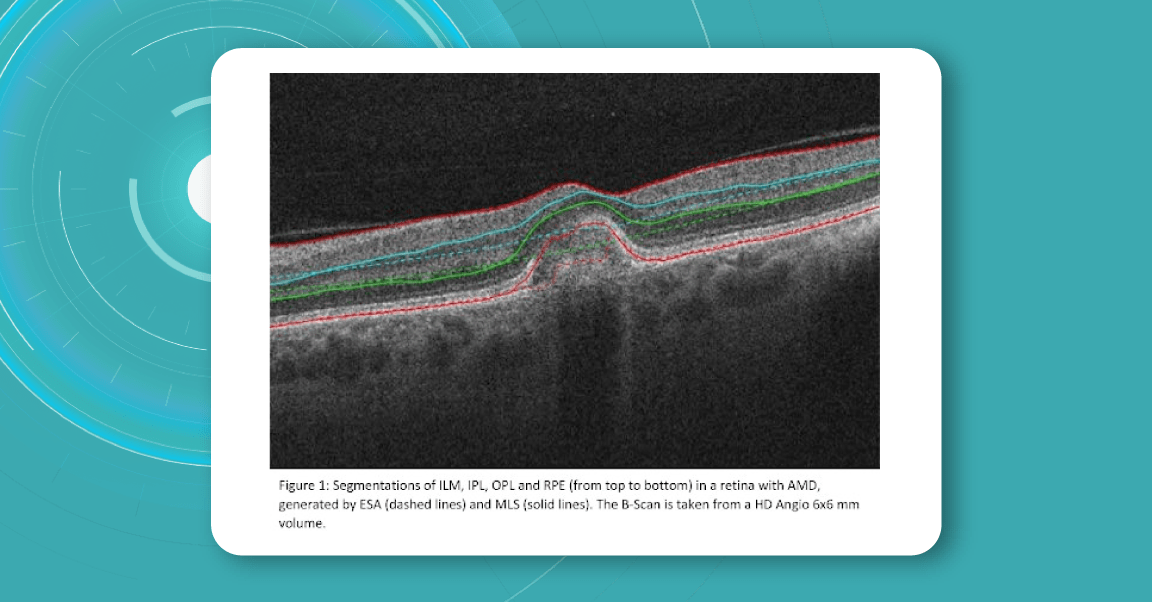
The existing segmentation algorithm (ESA) in CIRRUS estimates the positions of the inner plexiform layer (IPL) and outer plexiform layer (OPL) based on the internal limiting membrane (ILM) and retinal pigment epithelium (RPE). To improve the accuracy of the segmentation of these layers, a multi-layer segmentation algorithm (MLS) was introduced, it truly segments layers instead of estimating their position.
Heidelberg Engineering offers to learn about the following inner and outer retina layers on their website. There are 10 retina layers according to Heidelberg, and they are the following:
- ILM
- RNFL
- GCL
- IPL
- INL
- OPL
- ONL
- ELM
- PR
- RPE
- BM
- CC
- CS
Why accurate retina layer segmentation is important?
Retina layers segmentation helps eye care professionals to understand which pathology to consider in the first turn. For instance, changes in RPE and PR signify the development of Macular Degeneration.
Often such changes can also inform eye care specialists about the development of pathologies that lead to blindness, such as glaucoma, AMD, and Diabetic Retinopathy.
- Early Glaucoma Detection
Historically, evaluation of early glaucomatous change has focused mostly on optic disk changes. Modalities such as optical coherence tomography (OCT), confocal scanning laser ophthalmoscopy (HRT) or scanning laser polarimetry (GDx) with specially developed software algorithms have been used to quantitatively assess such changes. However, glaucomatous damage is primarily focused on retinal ganglion cells, which are particularly abundant in the peri-macular region (the only retinal area with a ganglion cell layer more than 1 layer thick), constituting, together with the nerve fiber layer, up to 35% of retinal macular thickness.
Therefore, glaucomatous changes causing ganglion cell death could potentially result in a reduction of retinal macular thickness. Indeed, by employing specially developed algorithms to analyze OCT scans, previous studies have reported that glaucoma, even during the early stage, results in the thinning of inner retinal layers at the macular region.
According to this study, the RNFL, GCL, and IPL levels out of all the retinal layers, the inner-most layers of the retina: the retinal nerve fiber layer (RNFL), ganglion cell layer (GCL), and inner plexiform layer (IPL) show the best discriminative power for glaucoma detection. Among these, the RNFL around the circumpapillary region has shown great potential for discrimination. The automatic detection and segmentation of these layers can be approached with different classical digital image processing techniques.

Test FDA-cleared AI for OCT analysis
- Detection of AMD
This first population-based study on spectral-domain optical coherence tomography-derived retinal layer thicknesses in a total of ∼1,000 individuals provides insights into the reliability of auto-segmentation and layer-specific reference values for an older population.
The findings showed a difference in thicknesses between early AMD and no AMD for some retinal layers, suggesting these as potential imaging biomarkers. When comparing layer thicknesses between early AMD and no AMD (822 eyes, 449 participants), the retinal pigment epithelium/Bruch’s membrane complex demonstrated a statistically significant thickening, and photoreceptor layers showed a significant thinning.
- Detection of DR
The depth and spatially resolved retinal thickness and reflectance measurements are potential biomarkers for the assessment and monitoring of Diabetic Retinopathy, one of the key reasons for blindness around the globe.
For instance, this study confirmed that decreased RNFL thickness and increased INL/OPL thickness in diabetics without DR or with initial DR suggest early alterations in the inner retina. On the contrary, the outer retina seems not to be affected at the early stages of DM. Automatic intraretinal layering by SD-OCT may be a useful tool to diagnose and monitor early intraretinal changes in DR.
Conclusion:
Retina layer segmentation is crucial for the accurate detection of pathologies in the eye, especially in the field of ophthalmology and medical imaging. Here are several reasons why it is important:
Precise Diagnosis: Retina layer segmentation provides a detailed map of the different retinal layers, which helps in the precise diagnosis of various eye conditions. It allows clinicians to identify the exact location of abnormalities, such as cysts, hemorrhages, or lesions, within the retina.
Quantitative Analysis: It enables quantitative analysis of retinal structures. By measuring the thickness, volume, and other characteristics of specific layers, clinicians can assess the severity and progression of diseases like diabetic retinopathy, macular degeneration, and glaucoma.
Early Detection: Some retinal pathologies manifest in specific layers of the retina before becoming visible on a fundus photograph. Retina layer segmentation can help detect these changes at an early stage, potentially leading to earlier intervention and improved outcomes.
Treatment Planning: Knowing the precise location of pathologies within the retina’s layers can aid in the planning of treatment strategies. For example, in cases of macular holes or retinal detachment, surgeons can use this information to guide their procedures.
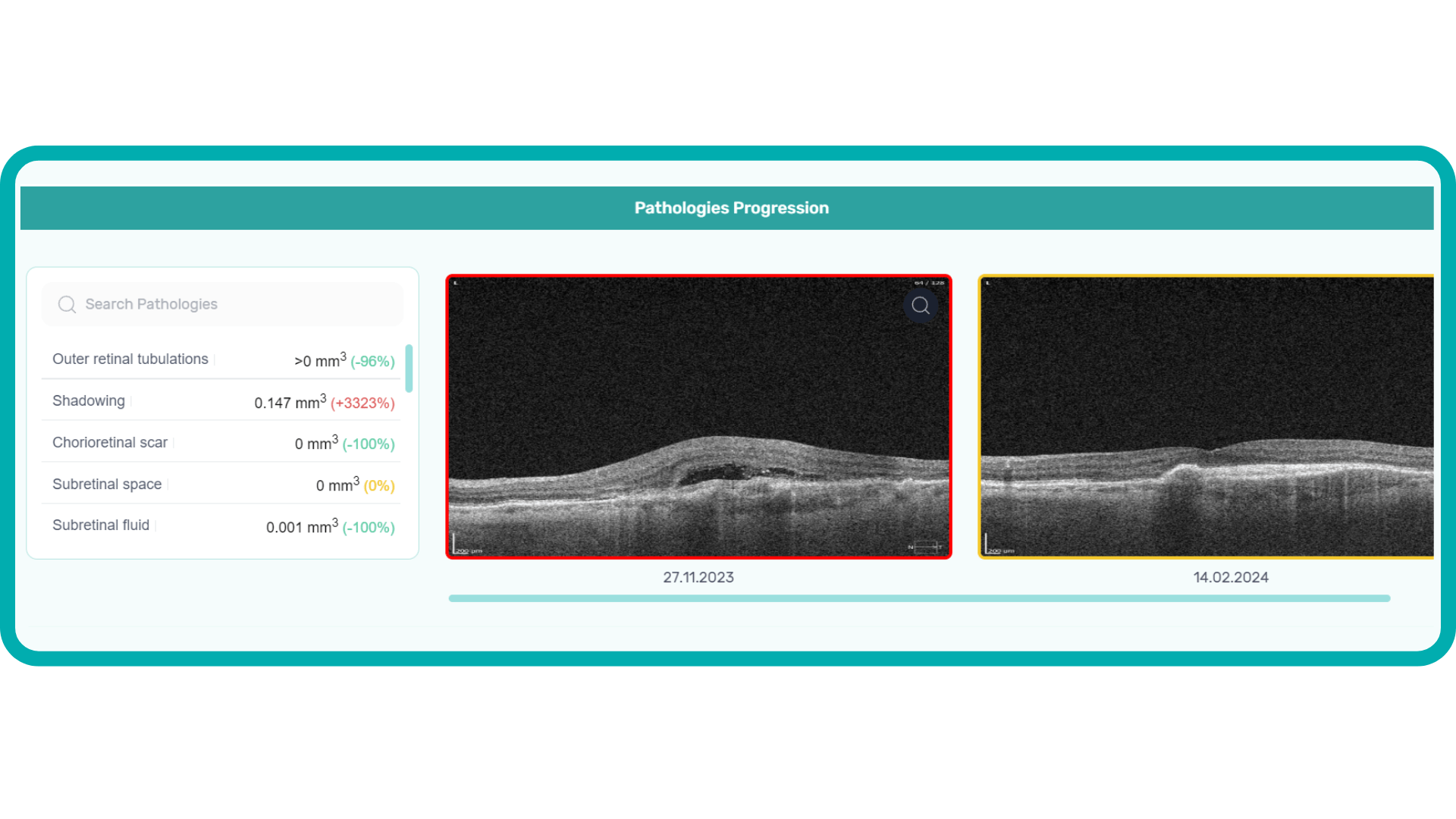
Monitoring Disease Progression: Retina layer segmentation is valuable for monitoring how retinal diseases progress over time. Changes in the thickness or integrity of specific layers can be tracked to assess the effectiveness of treatments or the worsening of conditions.
-

Altris AI for Buckingham and Hickson Optometry, the UK
 Altris Team
1 min.
Altris Team
1 min.Business case: Altris AI for Buckingham and Hickson Optometrists
The Client: Buckingham and Hickson is a family-run optometry practice that was established in 1960 in the United Kingdom. The optometry practice offers a number of services:
- Wide range of spectacle frames and lenses.
- Contact lenses.
- Glaucoma referral refinement.
- Cataract choice referral.
- OCT examination.
- NHS and private eye tests.

See how it works
FDA approved AI for OCT scan analysis
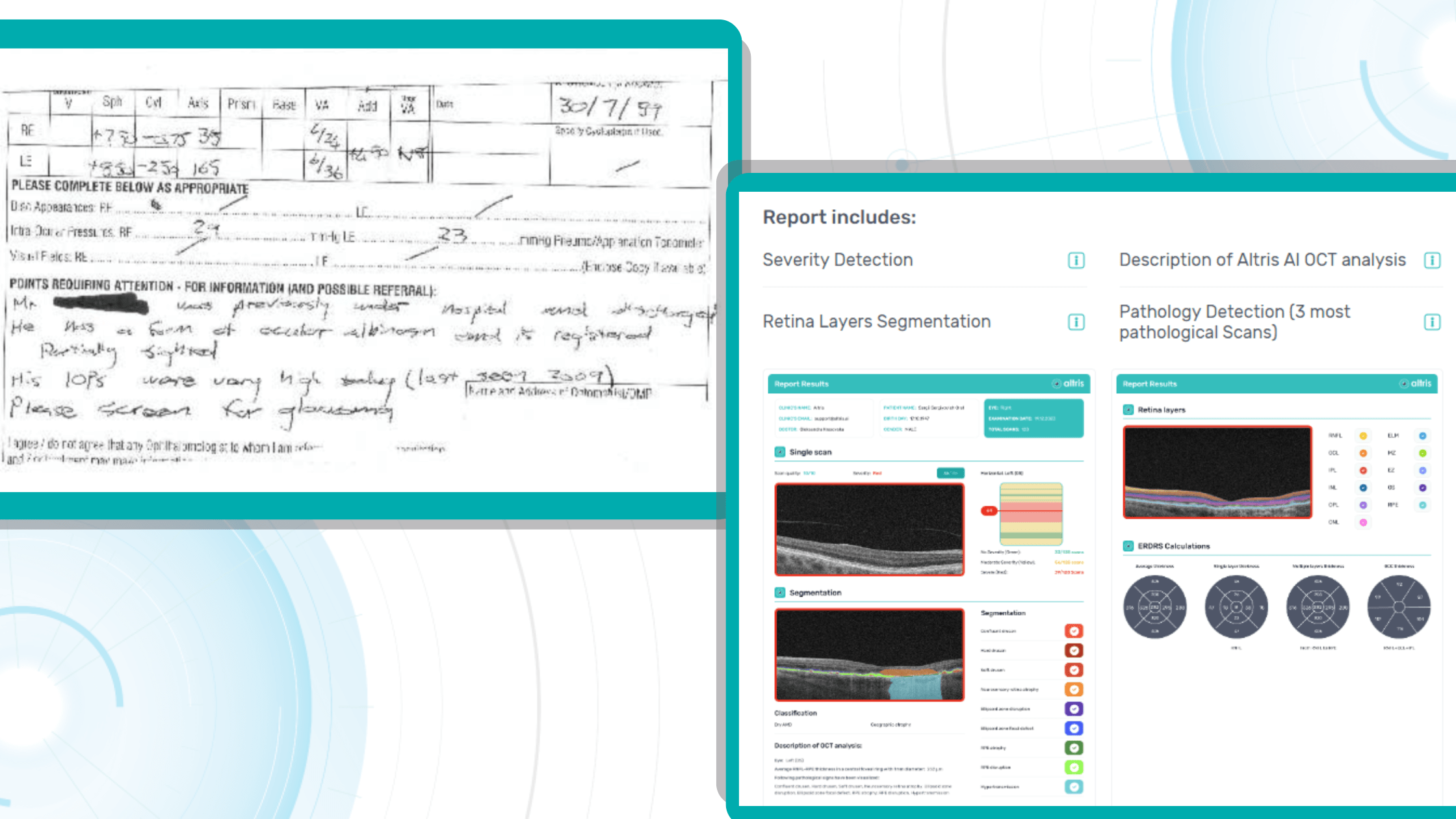
The challenge: The optometry owners wanted to test how Artificial Intelligence can assist them in OCT examination or, to be more precise, in providing a second opinion for OCT scans.
OCT examination is one of the best retina diagnostics methods, however in many cases OCT scan interpretation can be really challenging for several reasons:
- Variability in Anatomy: There is significant natural anatomical variation among individuals. What may be considered normal for one person may be abnormal for another. Eye care specialists need to account for these variations when interpreting OCT scans, but this often requires years of experience.
- Various Eye Conditions: Eye care specialists use OCT scans to diagnose and monitor a wide range of eye conditions, including macular degeneration, diabetic retinopathy, and retinal detachment, among others. Each of these conditions can manifest in different ways on OCT scans, making interpretation challenging.
- Progression Monitoring: Ophthalmologists often use OCT to monitor disease progression and the effectiveness of treatment. Tracking subtle changes over time can be difficult, as it requires precise comparisons of multiple scans.
- Artifacts: OCT scans are susceptible to artifacts, such as shadowing, motion artifacts, and signal dropout, which can obscure or distort the image. Recognizing and mitigating these artifacts is essential for accurate interpretation.
- Experience and Training: Accurate interpretation of OCT scans in optometry and ophthalmology requires specialized training and experience.
- Evolving Technology: OCT technology continues to advance, introducing new techniques and capabilities. Staying current with these advancements and understanding their clinical implications is an ongoing challenge for ophthalmologists.
-

AI for OCT analysis in optometry chains: 8 Reasons to invest
 Mark Braddon
5 min.
Mark Braddon
5 min.AI for OCT analysis in optometry chains
Optometry chains offer a wide range of eye care services, making it convenient for patients to access eye care locally.
However, the widespread accessibility of optometry chains has a reverse side for them. The shortage of employees, new unfamiliar equipment for diagnostics, and a large number of patients create an extremely challenging workflow for many optometrists. This, in turn, creates a number of challenges that can be more familiar to Optometry chains: low optometrist recruitment and retention, inconsistent quality of examination throughout the practices, lack of communication with patients, etc.
Automation of routine processes and digitalization have always served as answers to challenges like these in any industry, and healthcare is no exception. Luckily, automation of one of the most complex tasks for optometrists – OCT examination is already available to optometry chains with Artificial Intelligence (AI).
OCT proves to be one of the most efficient diagnostic tools for many modern top-notch optometry practices, however, mastering it requires skills and time. Artificial intelligence tools, such as AI for OCT analysis platform, can automate many routine processes which will have enormous benefits for any optometry chain. The top 8 benefits are the following:
-
#1 AI for OCT increases clinical efficiencies
Automating OCT scan analysis through AI reduces the time optometrists spend on image interpretation. This allows optometrists to focus on more complex cases, patient interactions, and personalized treatment plans. For any large optometry chain, saving time means providing more patients with high-quality service.
How does it work in practice?
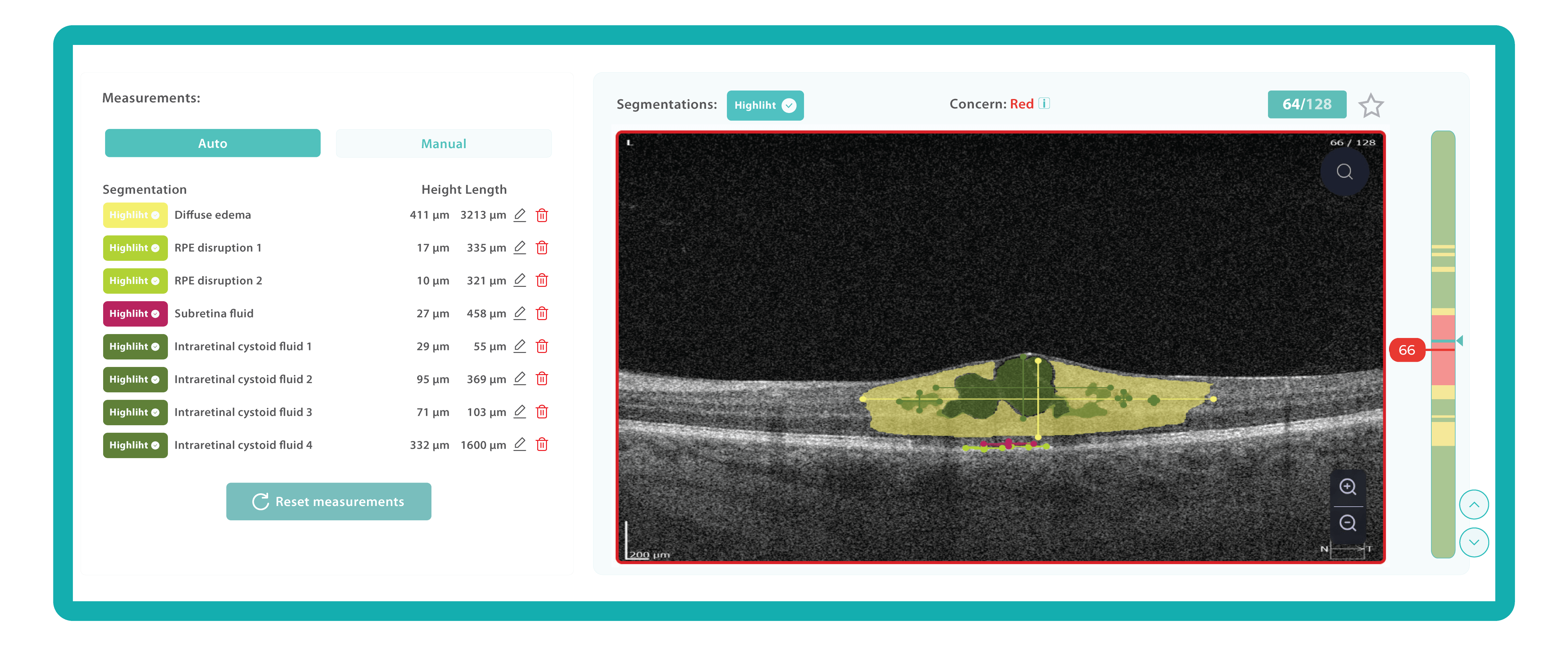
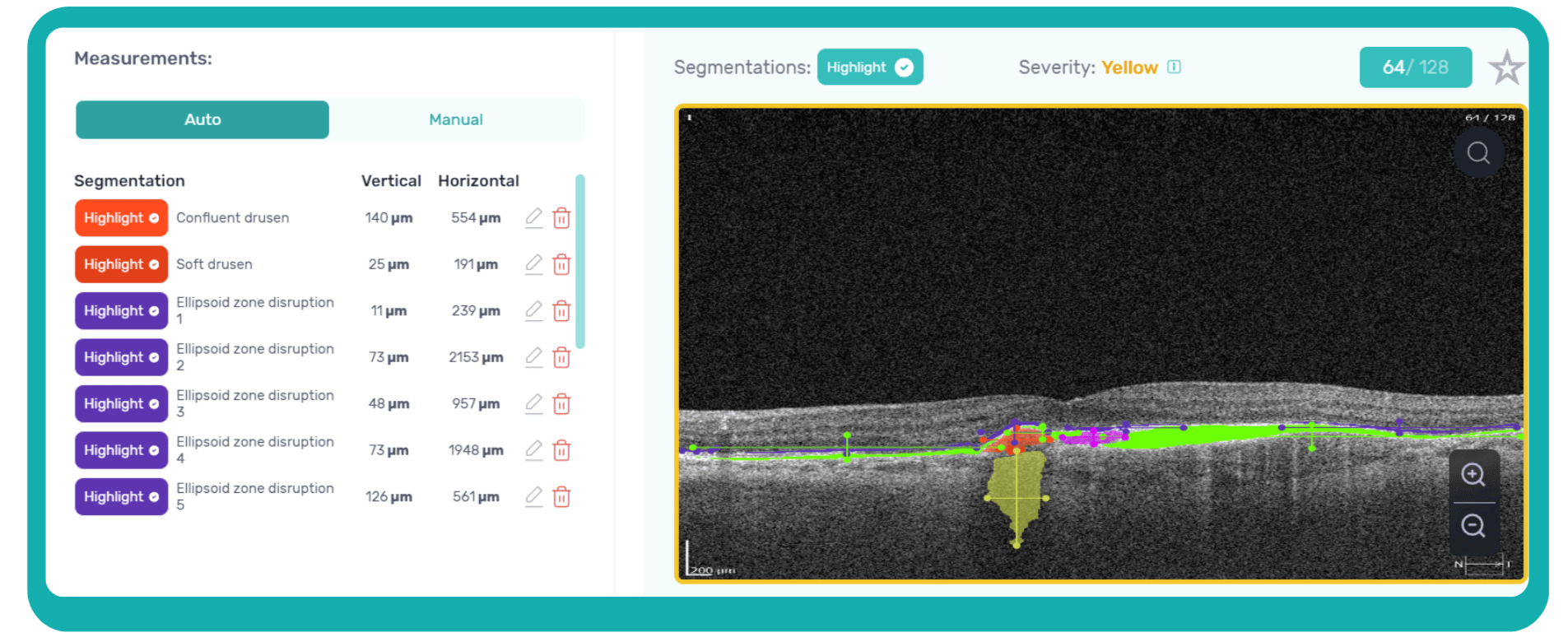
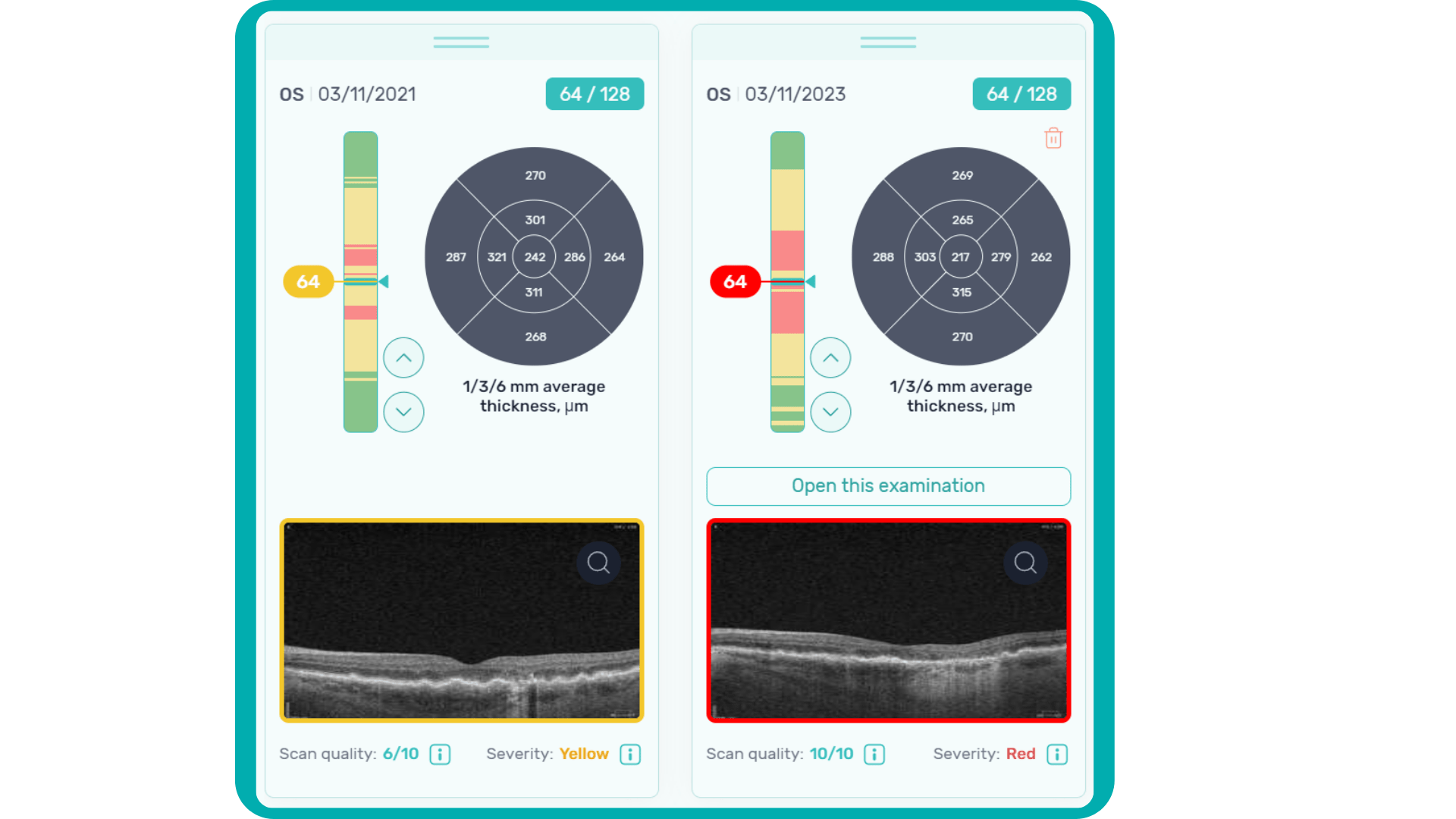
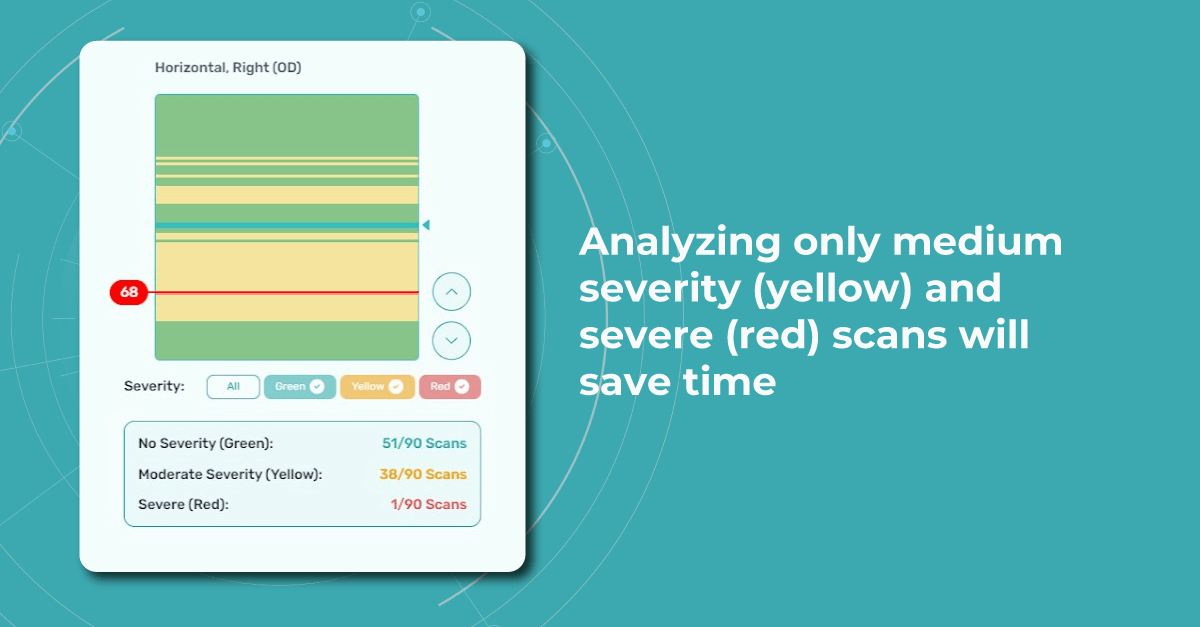
For instance, Altris AI has a severity grading of b-scans. Severity grading means that it is easy to see if the eye is healthy (removing any need to spend time interpreting) or highlight where the pathology is and the degree of severity.
- Green- no pathology detected
- Yellow- mild to medium level of severity
- Red – severe pathology detected
-
#2 AI for OCT provides consistently high standard of quality throughout the chain
AI algorithms provide consistent and standardized analysis regardless of the individual interpreting OCT scans. This reduces variability in diagnoses and ensures that patients receive uniform care across different clinics and practitioners within the optometry chain.
AI algorithms can analyze OCT scans with incredible precision and consistency. They can detect subtle changes in retinal structures that might be missed by human observers, leading to earlier and more accurate diagnoses of various eye conditions such as macular degeneration, glaucoma, diabetic retinopathy, and more.
This will help younger less experienced optometrists and will serve as a second opinion tool for more experienced specialists.

Test how Altris AI analyzes OCT
-
#3 AI for OCT enables better retention of employees
The shortage of optometrists in the world is staggering. 14 million optometry specialists are needed worldwide according to the WHO, while today there are only 331K ready to work.
It is equally difficult to hire and retain a good optometrist for a company in 2023. However, more and more young optometrists choose innovative businesses that use technology to improve the workflow. Top-notch equipment, convenient scheduling tools, and of course, Artificial Intelligence for OCT & fundus photo analysis might be the perks that will help optometrists to choose your optometry business.

Fresh from college optometrists feel more confident when they know that they will have a backup when reviewing OCT scans
-
#4 Reduced Workload Burden
Optometrists often have heavy workloads, and AI can help alleviate some of this burden by handling routine tasks like initial image analysis. This enables optometrists to spend more time on patient consultations and treatment planning.
According to a survey by the General Optical Council, 57% of optometrists worked beyond their hours in 2022. Optometrists were more likely to be working beyond their hours (60%) or finding it difficult to provide patients with the sufficient level of care they needed (34%) when compared to other registration types.
It is possible to outsource preliminary image analysis to Artificial Intelligence tools but communication and empathy are human tasks only.
-
# 5 AI promotes enhanced patient education
Let’s not forget about the patients. AI-generated OCT reports can help explain complex medical conditions to patients in a more understandable, visual way. After all 80% of all the information we receive is visual: imagine your optometrists not only telling but also showing what is going on with patients.
Comprehensive, color-coded OCT reports may improve patient education and engagement, leading to better treatment adherence and loyalty.
When patients don’t understand what they are paying for they are not likely to return for annual checkups. At Altris AI we created smart OCT reports that are comprehensible for patients as well as optometrists. We visualize all the pathologies and the patients can trace the dynamics of
#6 Reducing a clinical risk. No chances of getting a legal inquiry because of a pathology missed
Optometry chains can perform around 40K OCT scans a week. Statistically speaking, the chance of missing a minor early pathology is huge simply because of the big number.
With the double-check that AI for OCT scan analysis provides, It is not possible to wipe the risk out for 100%, but it is possible to diminish the risk to the absolute minimum.
For the optometry chain, it might mean no bad PR and weird stories in the papers and subsequently, a better brand image.
-
#7 AI makes early detection of pathologies possible on OCT
AI algorithms can identify early signs of eye diseases that might not be easily recognizable in their early stage. This early detection can lead to timely interventions, preventing or minimizing patient vision loss.
Glaucoma, Wet AMD, Diabetic Retinopathy, and genetic diseases are among the pathologies that lead to blindness if not detected in time. Detecting pathological signs and pathologies related to these disorders in time can literally save patients from future blindness.
Early detection of pathologies means that it is possible to stop or reduce the risk of total blindness which is the best result in any sense. Early detection will allow optometrists to give valid recommendations, and advise on dieting and supplements right at the optical store.
-
#8 Competitive Edge
AI is a buzzword, and it’s not accidental. All major players understand its enormous value and invest in it. During the last presentation, the CEO of Google said “AI” 140 times, and let’s be honest, it is not to show off. It is because AI can actually make changes in business: automation of repetitive processes, workflow optimization, and human error reduction.
Adopting AI technology for OCT analysis showcases the optometry chain’s commitment to staying at the forefront of technological advancements in healthcare. Gaining a real competitive edge is another big goal.
This can attract patients who value cutting-edge approaches to diagnosis and treatment. A younger generation of patients are curious about new technologies, and this can be an additional lead magnet for them.

Conclusion
Incorporating AI for OCT analysis into optometry chains can enhance patient outcomes, make the workflow more efficient, and improve the performance of each optometry center. However, it’s important to ensure that the AI systems are properly validated, integrated into clinical workflows, and monitored to maintain their accuracy and effectiveness. More than that, it should complement, not replace, the expertise of optometrists. The technology should be used as a tool to aid optometrists and make OCT examination more effective.
-
-

Normative Database in OCT: Limitations and AI Solutions
 Maria Martynova
06.09.20236 min read
Maria Martynova
06.09.20236 min readThe first normative database for OCT was created in the early 2000s and were based on small studies of mostly white patients. However, as OCT technology has evolved, so too have the normative databases. Recent databases are larger and more diverse, reflecting the increasing ethnic and racial diversity of the population.

FDA-cleared AI for OCT
Make your eye care business technological
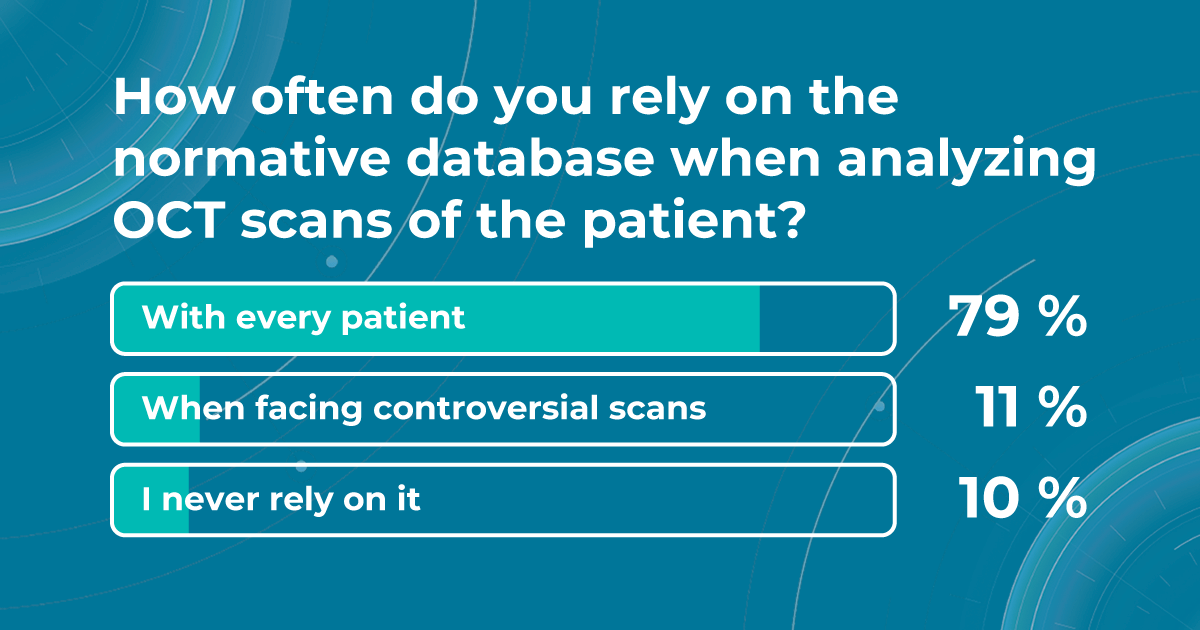
Nowadays, eye care specialists use normative database to compare the characteristics of a patient to a population-wide norm. This allows them to quickly and easily assess whether a patient’s retinal dimensions fall within normal limits. According to our survey, 79% of eye care specialists rely on the normative databases for OCT verdict with every patient.
However, despite the fact that normative databases are very widespread among specialists worldwide, they are not perfect. They can be affected by factors such as age, gender, axial length, and refractive error.
They can be influenced by low image quality due to different eye pathologies. It is essential to be aware of these limitations when interpreting normative data OCT parameters. That is why, in this article, we will discuss the benefits of the collaboration between AI decision-making tools and normative databases to improve patient outcomes.
What is a normative database, and is there a difference between normative databases for different devices?
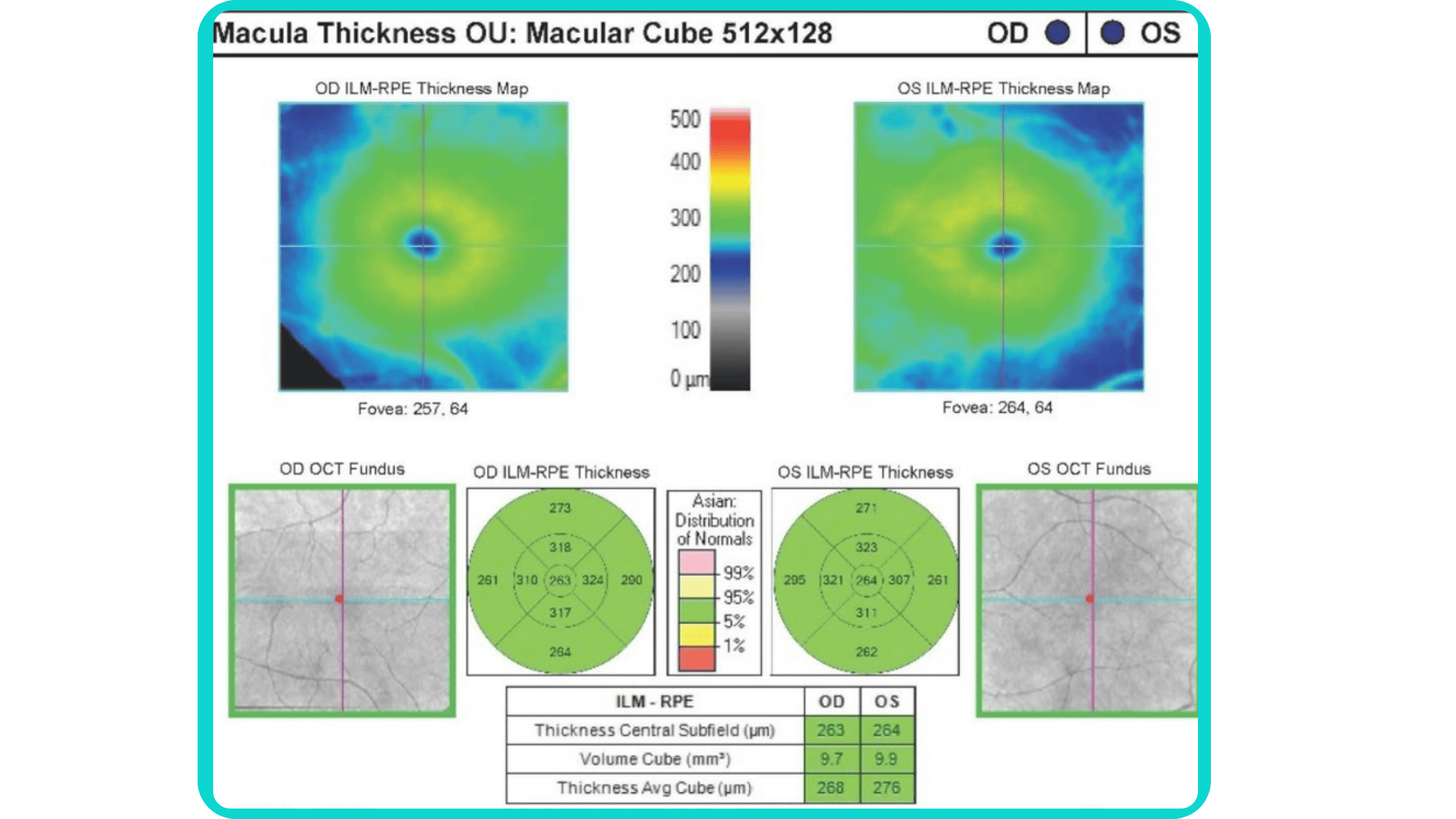
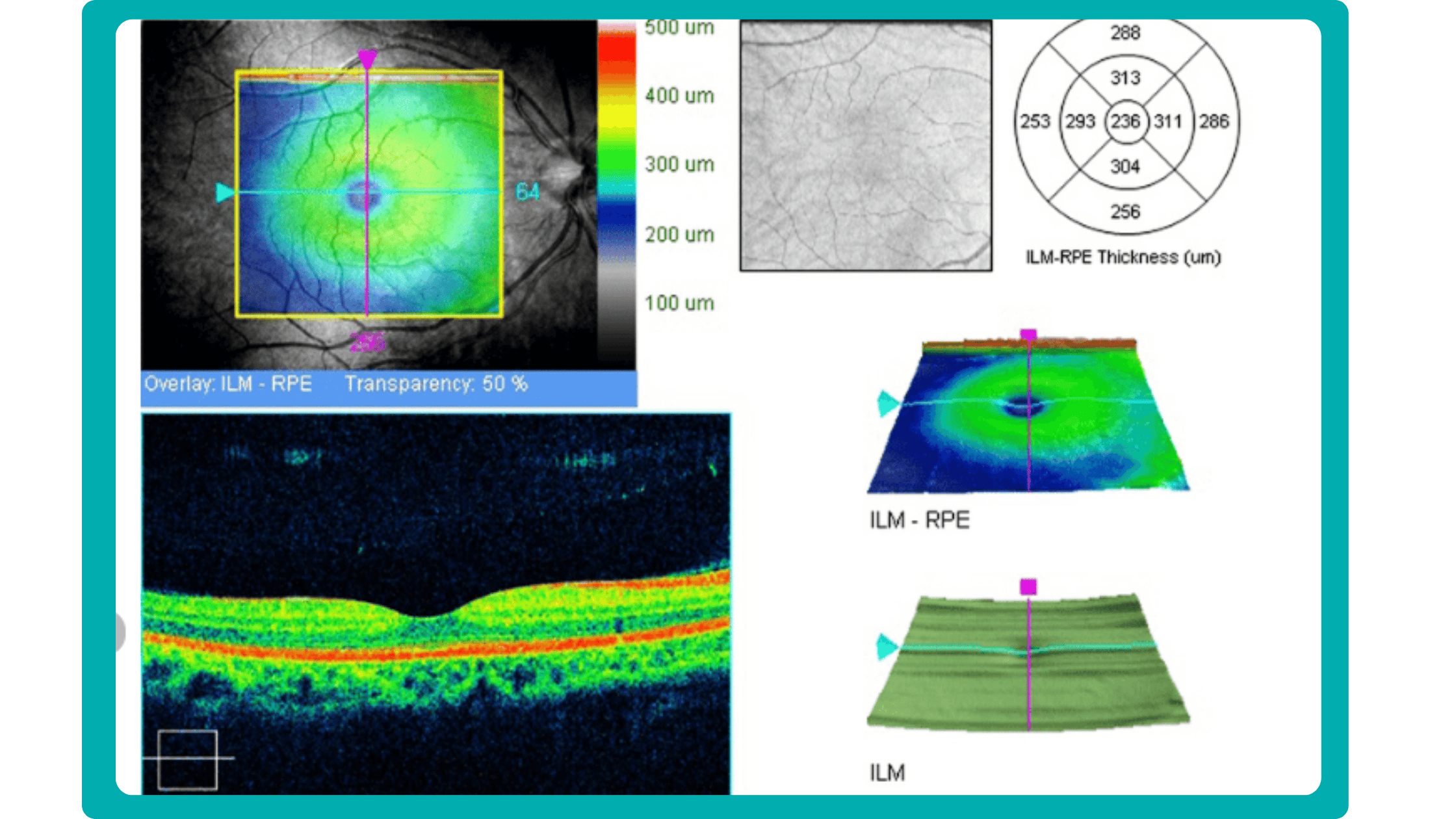
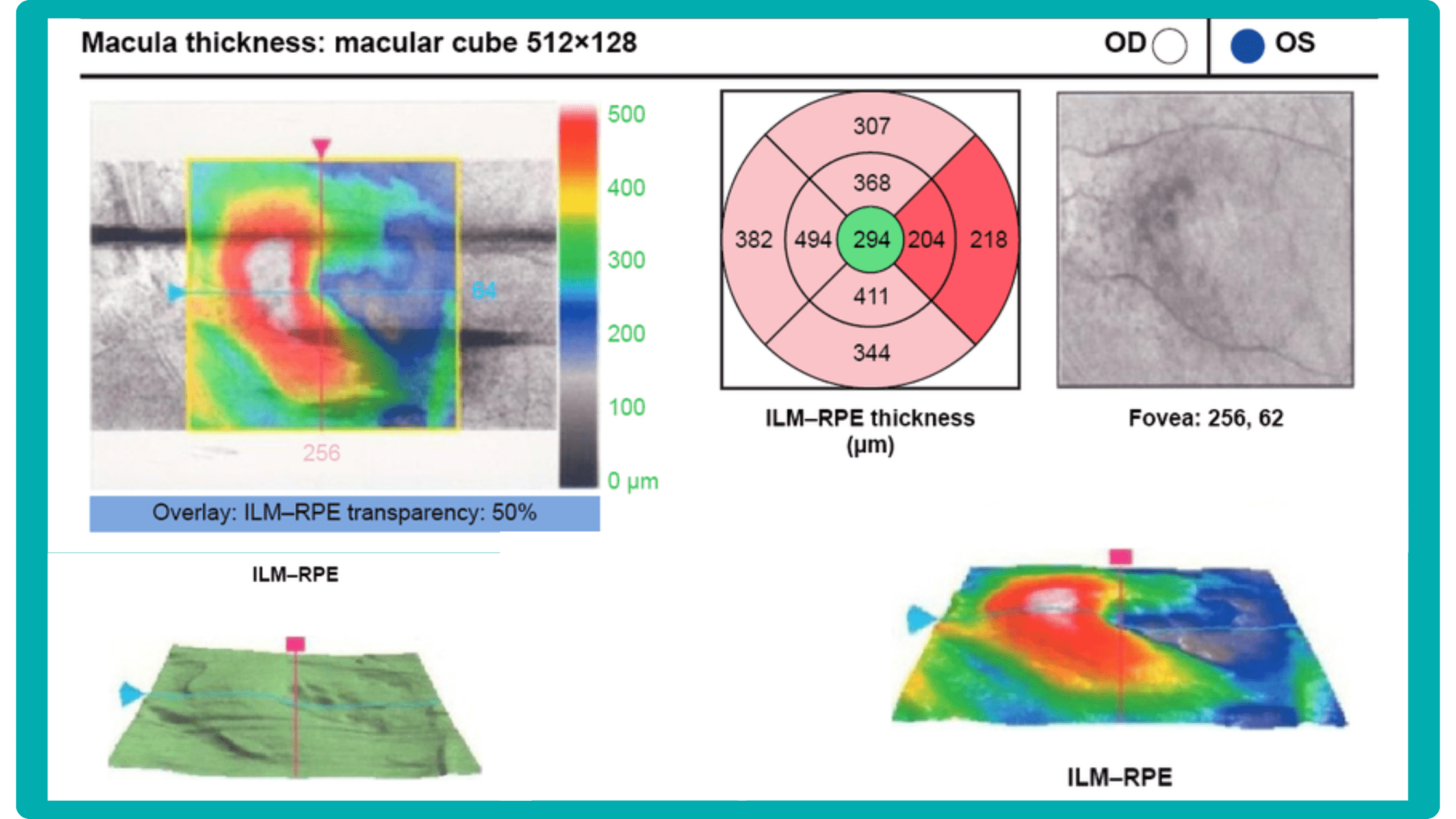
Before diving into the subject of the benefits and limitations of normative databases, we would like to remind you what a normative database is. From the moment of its invention, the OCT exam has rapidly gained widespread adoption and has become indispensable in the eye care practice. Critical to this success has been the ability of software to automatically produce important measurements, such as the thickness of the peripapillary retinal nerve fiber layer (RNFL) in tracking glaucoma progression or the total retinal thickness in the assessment of macular diseases.
In order to accurately interpret OCT scans, normative databases were created. These databases are now built into almost all commercial OCT devices, allowing eye care specialists to view colored reports and progression maps that assist in the rapid recognition and tracking of pathology.
Summing up, a normative database for OCT is a set of data that provides references for OCT thickness measurements in a healthy population. These databases are used to compare the OCT measurements of your patient to a population-wide norm.
Here are some of the OCT parameters that are commonly measured and compared to normative databases:
- Retinal nerve fiber layer (RNFL) thickness: the RNFL is a retinal layer that is measured around the optic nerve. This measurement is important for diagnosing optic nerve atrophy.
- Macular thickness: the macula is responsible for sharp central vision.
- Ganglion cell complex thickness: the ganglion cell complex is a group of cells in the retina that are responsible for transmitting visual information to the brain.
- Cup-to-disc ratio, neuroretinal rim, and other optic nerve parameters: are very important for diagnosing glaucoma and other optic nerve pathologies
These are just a few of the OCT parameters that are commonly measured in normative databases. The specific parameters that are measured can vary depending on the type of OCT device and the clinical application.
In addition, different OCT devices can have different measurement capabilities and resolutions. For example, a device that uses time-domain OCT (TD-OCT) technology may have a lower resolution than a device that uses spectral-domain or swept-source OCT (SD or SS-OCT) technology. This means that the normative database for a TD-OCT device may not be as accurate as the normative database for an SD or SS-OCT device.
What is more, the normative database for a particular device may be based on a specific population of patients. What are the benefits and limitations of normative databases?
Now that we have highlighted different aspects of the normative database definition let us discuss the benefits and limitations of this tool. Normative databases can sometimes be very helpful for eye care specialists in diagnosis, decision-making, and creating a treatment strategy for eye diseases such as glaucoma and macular degeneration.
- The measurement provided by the normative database can be used as a baseline for tracking a patient’s response to medication or other treatment. Eye care specialists can track changes between a few visits and determine the impact on the patient.
- Normative databases show deviations from the norm, which may be a reason for a more comprehensive examination.
- Eye care specialists can also use normative databases to compare the results of different OCT devices. This can help to ensure that they are using the most accurate device for their patients.
There are still challenges that must be overcome to develop normative databases sufficient for use in clinical trials. That is why current normative databases also have a lot of limitations.
Does not detect pathology
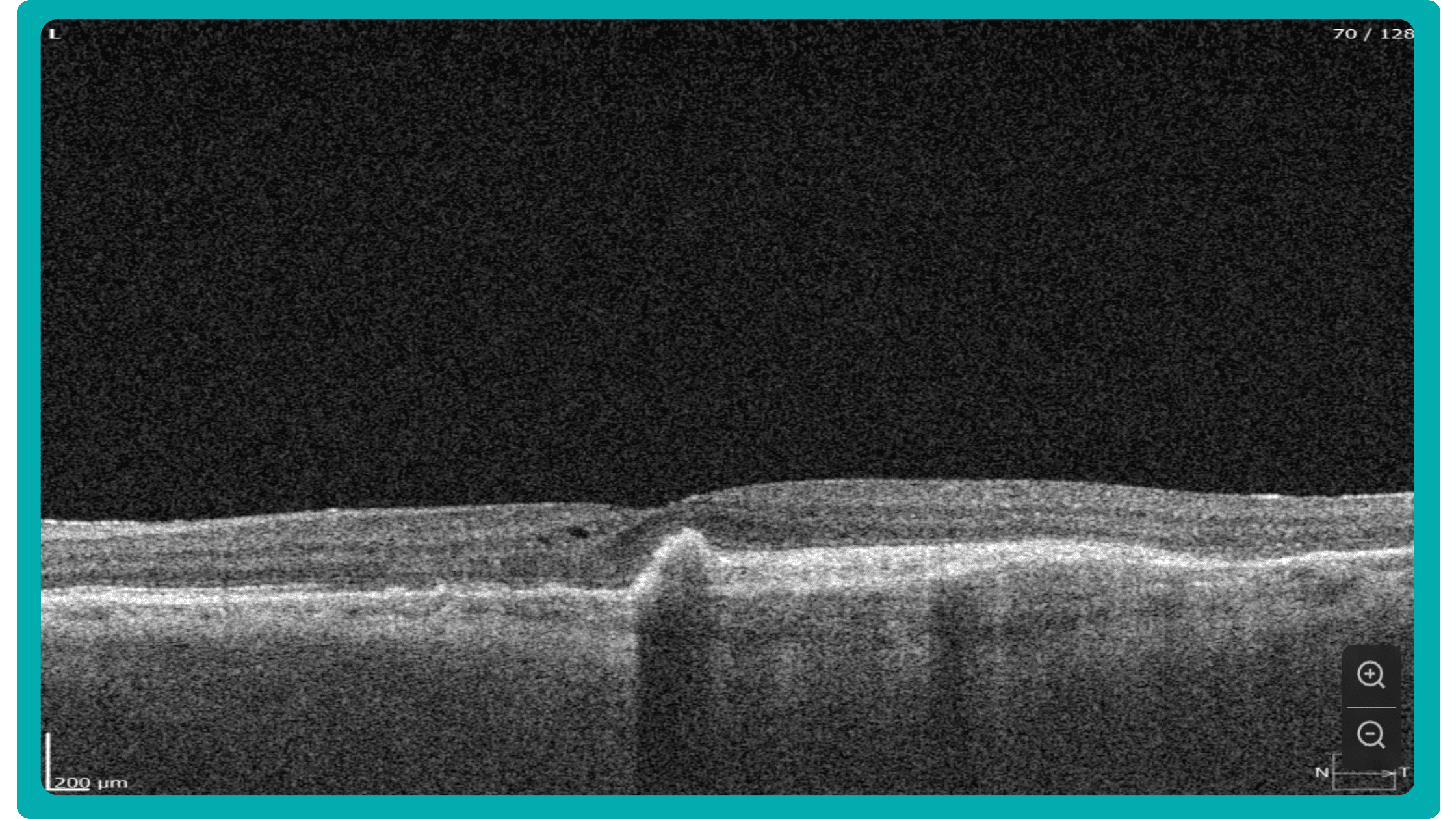
The normative database works only with the thickness of the retina and does not detect what is inside the retina. Therefore, it cannot detect all pathologies where there is no change in retinal thickness. In the early stages, these are absolutely all diseases. We can see deviations from the normative base only when the disease progresses to a later and more severe stage when the retinal thickness decreases or increases.
Limited diversity
Normative databases can be limited by factors like age, gender, and ethnicity of the population used to create them. This can result in reduced accuracy for patients who are not well-represented in the database.
Population variation
Even healthy patients can have some anatomical variations that fall within the range of normal. These variations may be falsely flagged as abnormalities when compared to the database.
How Altris AI platform can complement the information provided by the normative database
Normative databases in OCT play a crucial role in aiding diagnosis and treatment planning, but they also have limitations related to representation, disease progression, and data quality. Eye care specialists need to interpret the results in the context of the patient’s individual characteristics and other clinical information, using additional tools for scan interpretations.
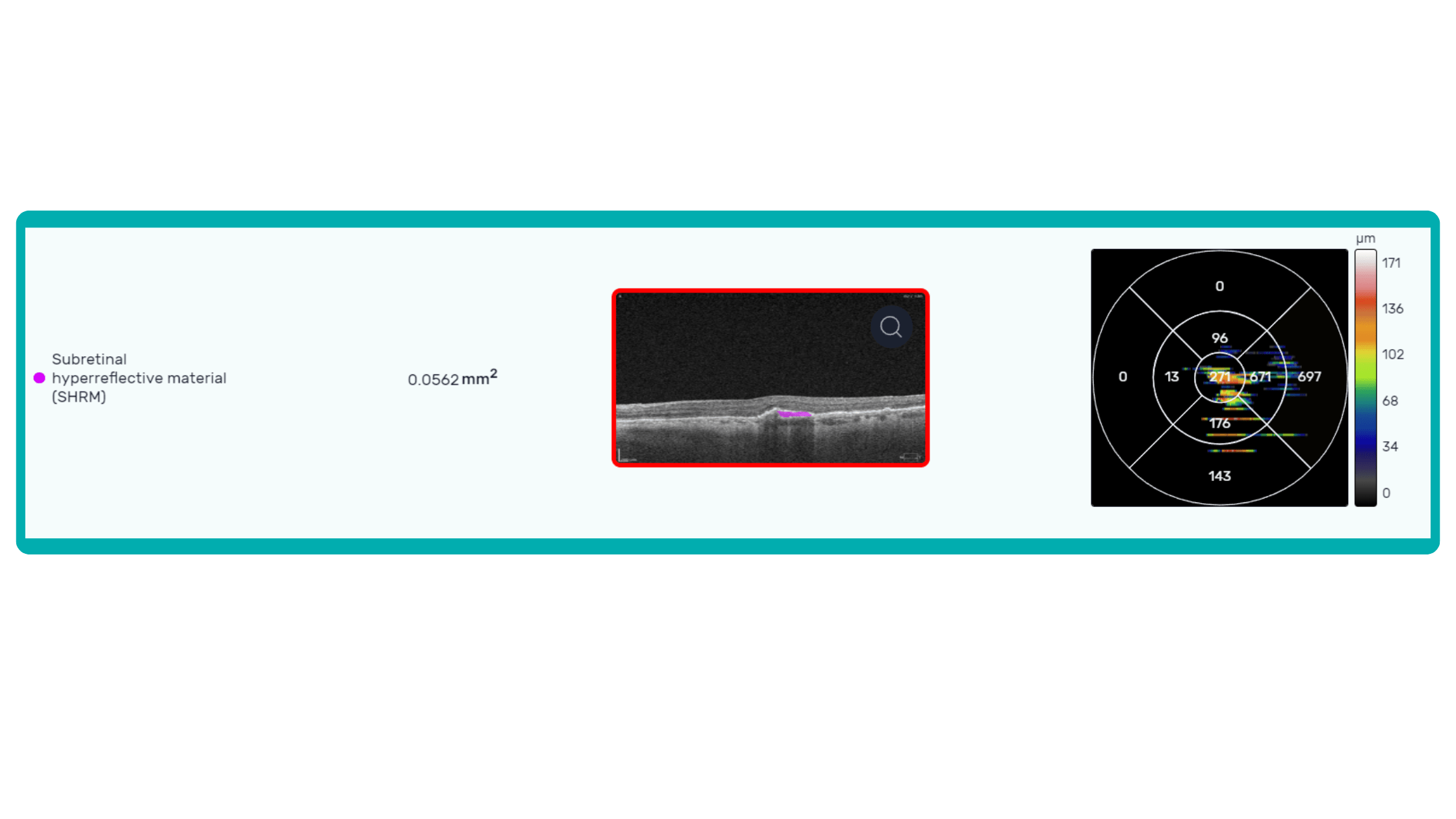
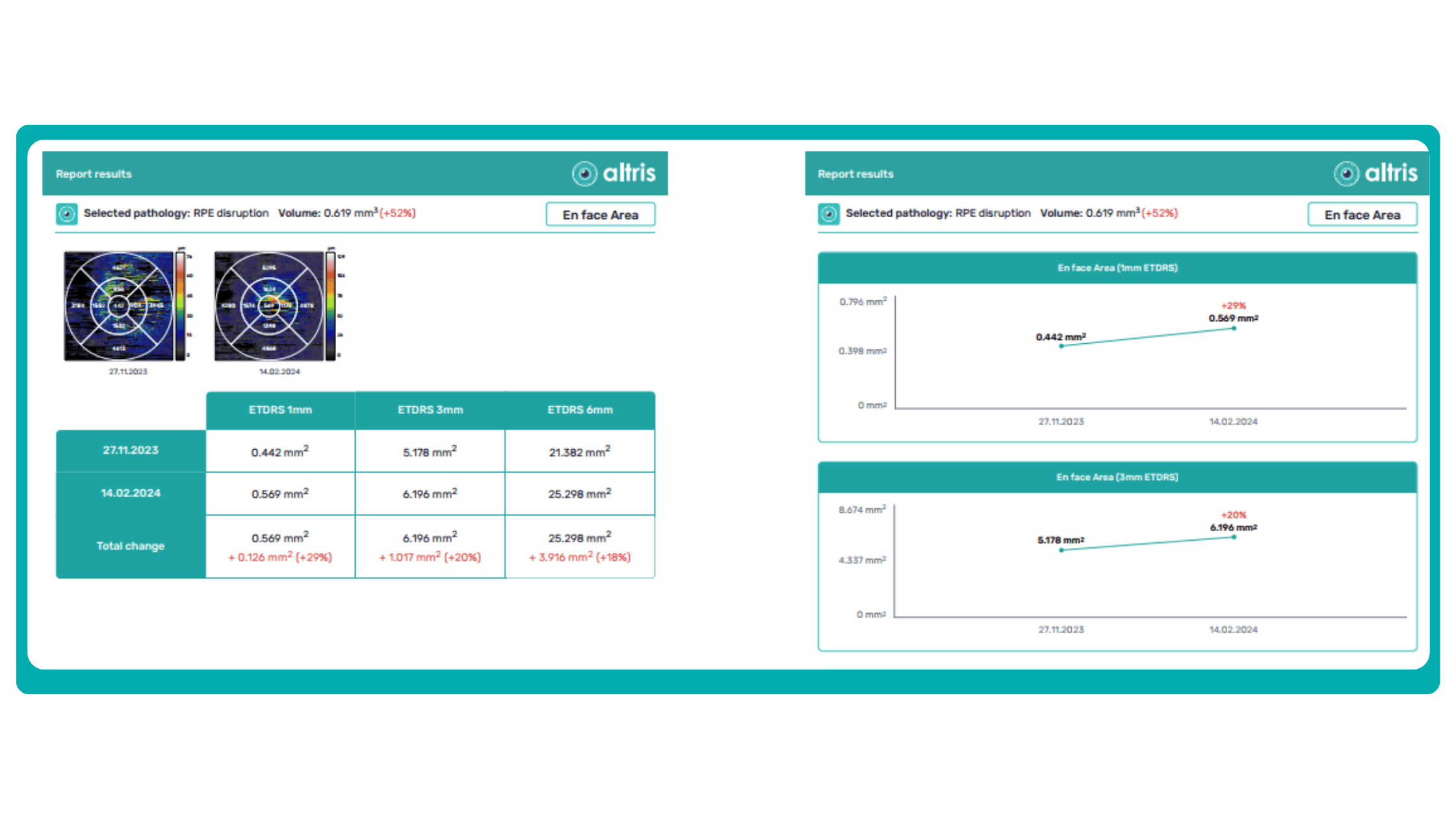
Sometimes, low-quality OCT scans can be inaccurately interpreted by the eye care specialist, and the normative database can showcase inaccurate measurements. Altris AI platform detects low-quality scans automatically and warns about the possibility of inaccurate results. In addition, the platform automates the detection of 70+ pathologies and pathological signs. Once the user uploads the scan, they can see visualized and highlighted pathological areas and pathology classification that the algorithm has detected. The user can also calculate the area and volume of detected biomarkers.
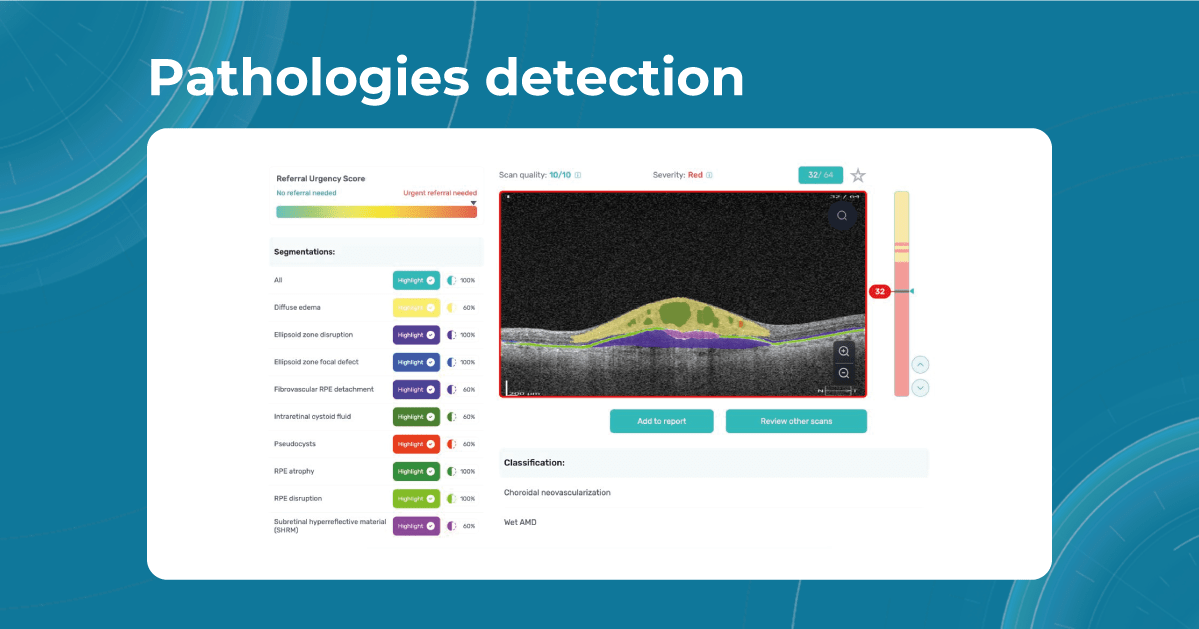
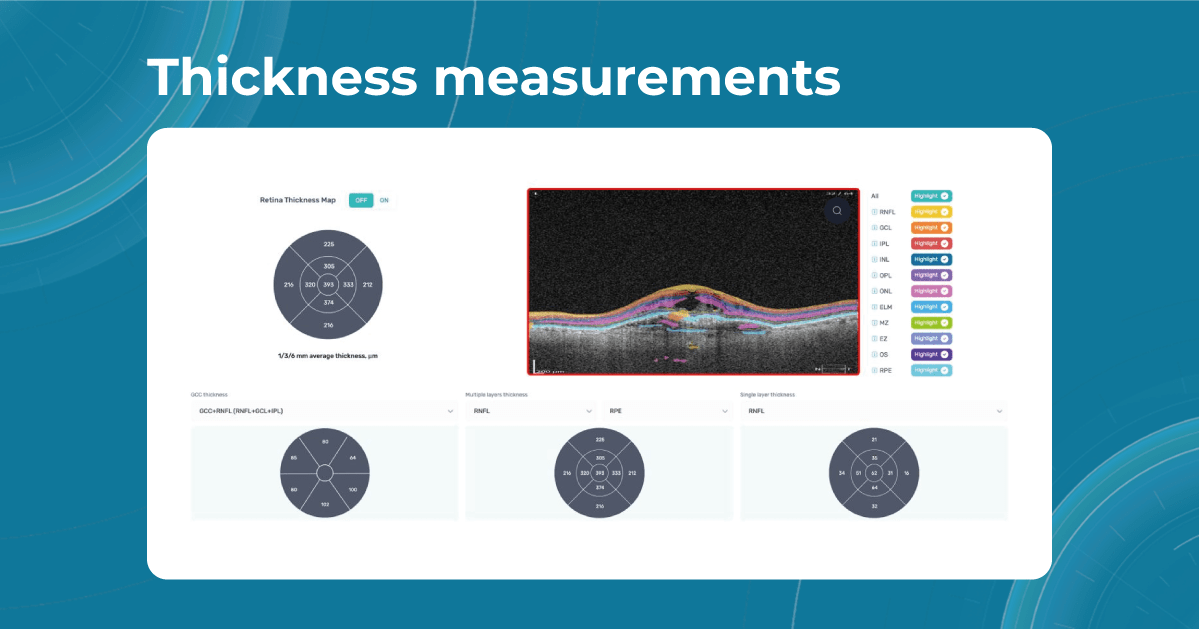
Artificial intelligence-based tools for OCT interpretation used along with normative databases can play a crucial role in clinical eye care. Altris AI, for example, can provide eye care specialists with additional and more precise information about separate retinal layer thickness. The system analyzes the thickness of each retina layer or several layers combined.
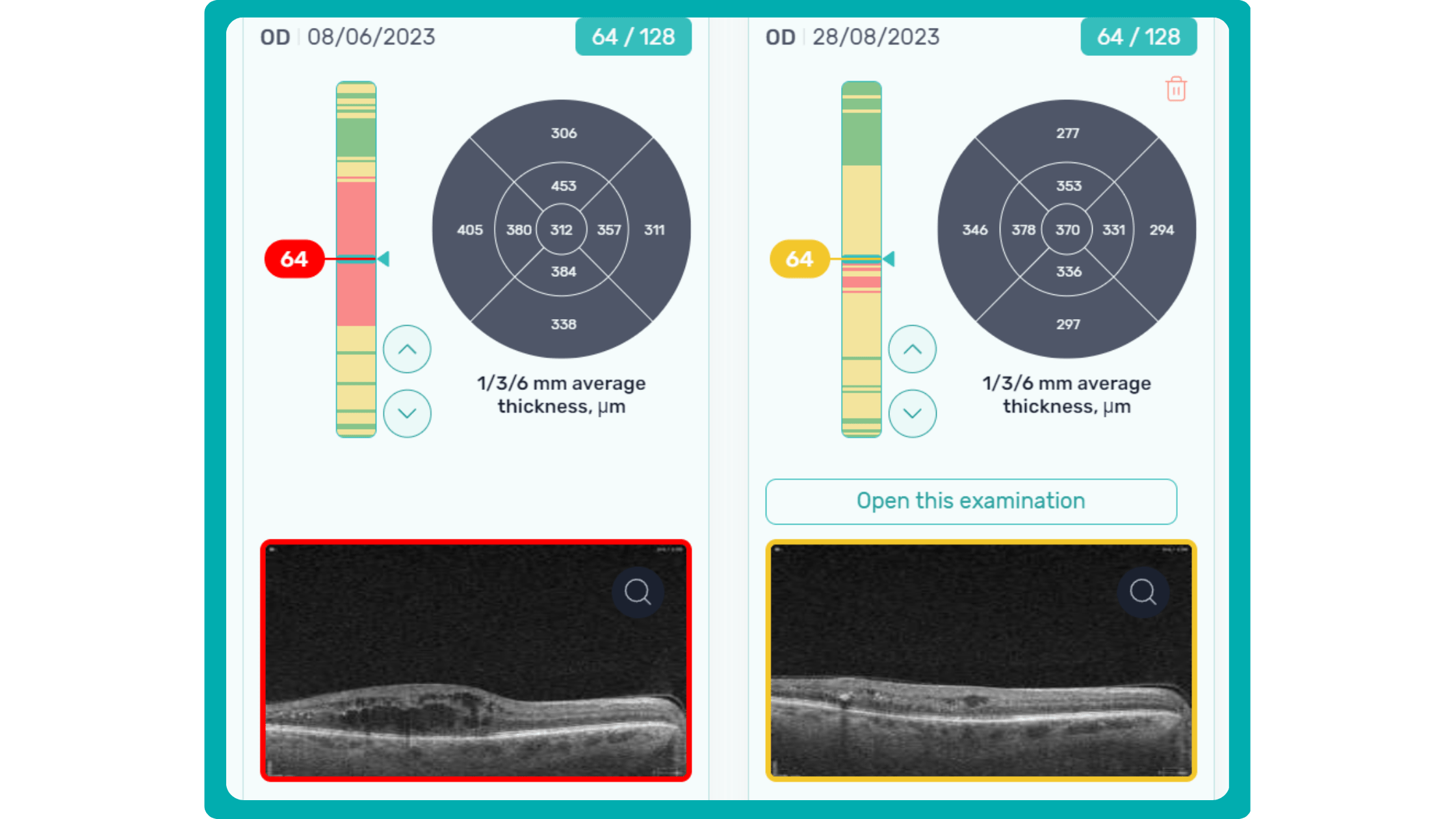
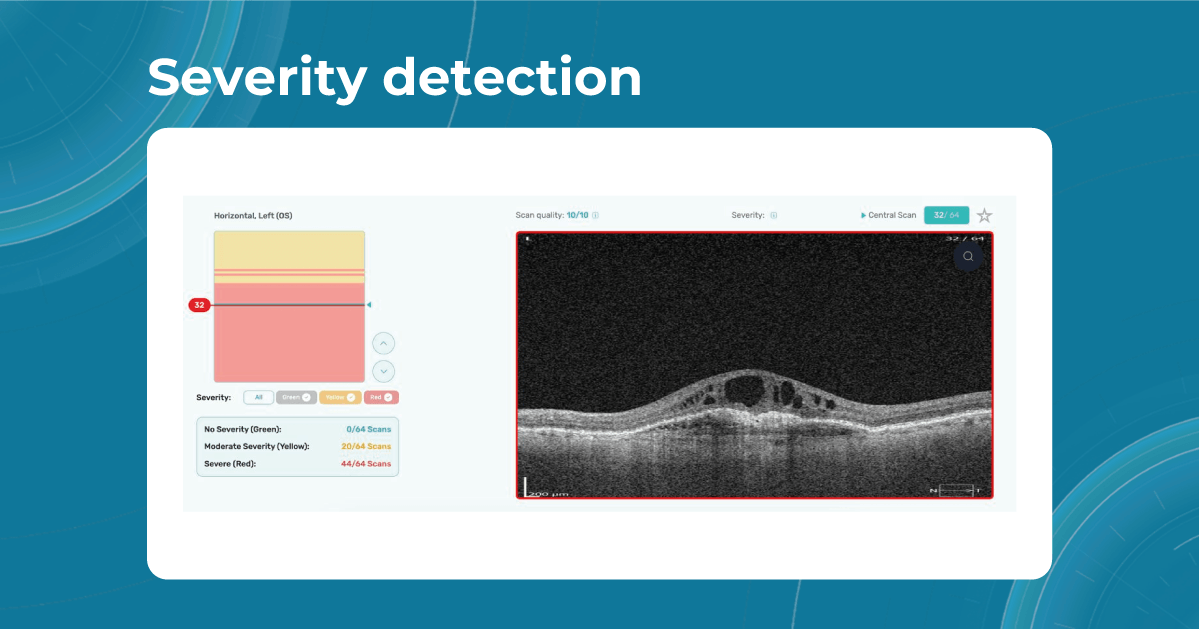
While normative databases provide information only about the thickness, AI tools equipped with deep learning models can detect pathological signs in OCT scans that might be missed by the normative database or the human eye, enhancing diagnostic accuracy. Altris AI algorithm classifies the OCT scans based on the degree of pathology found. It can distinguish green concern, which indicates normal retina, yellow – moderate with slight deviations, and red concern, which means high severity level.
Summing up
Despite their limitations, normative databases are an essential tool for the clinical use of OCT. They provide a valuable reference point for assessing patients and can help to identify some diseases. However, the normative database measures only the thickness, which is not enough to accurately diagnose the patient and create a treatment plan.

FDA-cleared AI for OCT
Make your eye care business technological
That is why incorporating AI into OCT interpretation streamlines the decision-making process. By automating the initial analysis of OCT scans, specialists can focus their attention on more complex cases, making the best use of their skills and experience. Moreover, embracing AI technologies empowers eye care specialists to personalize patient care with greater precision.