

Maria Martynova
Reading time
10 min read
Glaucoma’s silent progression highlights a challenge we all face as clinicians. Millions of individuals remain at risk for irreversible vision loss due to undiagnosed disease – 50% or more of all cases. This emphasizes our responsibility to enhance early detection strategies for this sight-threatening condition.
Existing clinical, structural, and functional tests depend on both baseline exams and the need to observe changes over time, delaying the assessment of treatment effectiveness and the identification of rapid progression.
In this article, we will consolidate our knowledge as eye care professionals about Glaucoma, explore current clinical detection practices, and discuss potential areas to optimize early Glaucoma detection.
FDA-cleared AI-powered OCT Glaucoma Risk Assessment
What we know about Glaucoma
Glaucoma is a complex neurodegeneration fundamentally linked to changes occurring in two locations: the anterior eye (elevated pressure) and the posterior eye (optic neuropathy). Factors influencing glaucoma development include:
- age,
- ethnicity,
- family history,
- corneal thickness,
- blood pressure,
- cerebrospinal fluid pressure,
- intraocular pressure (IOP),
- and vascular dysregulation.
Early stages of Glaucoma are often asymptomatic, highlighting the importance of comprehensive eye exams, even without apparent vision issues. Current diagnostic criteria are insufficient and lack markers of early disease.
Glaucoma is broadly divided into primary and secondary types, with primary open-angle Glaucoma (POAG) representing approximately three-quarters (74%) of all glaucoma cases.
Primary glaucomas develop independently of other eye conditions, while secondary glaucomas arise as a complication of various eye diseases, injuries, or medications.
POAG is characterized by an open iridocorneal angle, IOP usually > 21 mmHg, and optic neuropathy. Risk factors include age (over 50), African ancestry, and elevated IOP. While IOP is a significant factor, it’s unpredictable – some patients with high IOP don’t develop Glaucoma, and some glaucoma progresses even at normal IOP.
Normal-tension Glaucoma (NTG) shares POAG’s optic nerve degeneration but with consistently normal IOP levels (<21mmHg). Vascular dysregulation and low blood pressure are risk factors. While rarer than POAG, IOP lowering can still be beneficial.
Primary Angle-Closure Glaucoma (PACG) is caused by narrowing the iridocorneal angle, blocking aqueous humor flow. More common in East Asian populations, it can be acute (severe symptoms, IOP often > 30mmHg) or chronic.
Secondary glaucomas are caused by underlying conditions that elevate IOP. Examples include pseudoexfoliative, neovascular, pigmentary, and steroid-induced Glaucoma.
Age is a central risk factor for glaucoma progression, linked to cellular senescence, oxidative stress, and reduced resilience in retinal ganglion cells and the trabecular meshwork. Intraocular pressure (IOP) remains the most significant modifiable risk factor. Understanding individual susceptibility to IOP-related damage is crucial. Existing IOP-lowering treatments have limitations in both efficacy and side effects.
Glaucoma has a strong genetic component, with complex interactions between genes, signaling pathways, and environmental stressors. For now, we know that mutations in each of three genes, myocilin (MYOC), optineurin (OPTN), and TANK binding kinase 1 (TBK1), may cause primary open-angle Glaucoma (POAG), which is inherited as a Mendelian trait and is responsible for ~5% of cases (Mendelian genes in primary open-angle Glaucoma).
More extensive effect mutations are rare, and more minor variants are common. Genome-wide association studies (GWAS) reveal additional genes potentially involved in pressure sensitivity, mechanotransduction, and metabolic signaling.
Recent research also suggests a window of potential reversibility even at late stages of apoptosis (a programmed cell death pathway, which is likely the final step in RGC loss). Cells may recover if the harmful stimulus is removed. This offers hope that dysfunctional but not yet dead RGCs could be rescued.
The Challenges of Early Glaucoma Detection
One of the most insidious aspects of Glaucoma is its largely asymptomatic nature, especially in the early stages. This highlights the limitations of relying on symptoms alone and underscores the importance of proactive detection strategies.
Relying on intraocular pressure (IOP) as a stand-alone glaucoma biomarker leads to missed diagnoses, especially in patients with normal-tension Glaucoma. Structural changes, such as optic disc cupping, also lack the desired sensitivity and specificity for early detection.
Optic nerve head evaluations remain subjective, with studies indicating that even experienced ophthalmologists can underestimate or overestimate glaucoma likelihood.
According to the research, even experienced clinicians can have difficulty evaluating the optic disc for Glaucoma. Both trainees and comprehensive ophthalmologists have been found to underestimate glaucoma likelihood in approximately 20% of disc photos. They may also misjudge risk due to factors like variations in cup-to-disc ratio, subtle RNFL atrophy, or disc hemorrhages.
Current Glaucoma Diagnosis in Clinical Practice
Eye care professionals typically encounter new glaucoma diagnoses in one of two ways:
- Firstly, during routine preventive examinations. A patient may come in for various reasons, including work requirements, and be found to have elevated intraocular pressure. This finding prompts further evaluation, potentially leading to a glaucoma diagnosis.
- Secondly, it is a finding in older patients (often over 50-60). A patient may present with significant vision loss in one eye, and examination reveals Glaucoma. Unfortunately, vision loss at this stage is often irreversible.
Alternatively, a patient may seek care for an unrelated eye problem. During the comprehensive examination, the eye care professional may discover changes suggestive of Glaucoma.
As it is statistically prevalent, we most often work with primary Glaucoma, where no other underlying eye diseases are present. Functional changes, specifically as seen on visual field testing, help diagnose and stage glaucoma. During the test, a patient indicates which light signals are visible within their field of vision, building a map of each eye’s visual function.
Vision Field Test for Glaucoma Detection
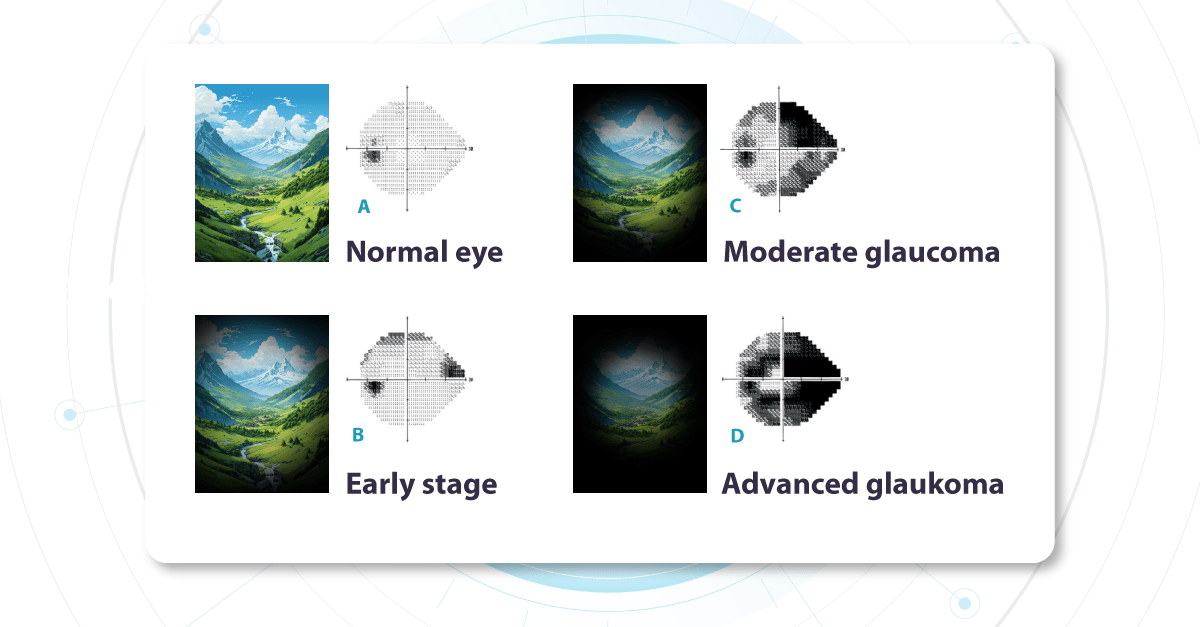
The optic nerve (a nerve fiber layer of the retina consisting of the axons of the ganglion neurons coursing on the vitreal surface of the retina to the optic disk) transmits visual information from the retina to the brain. Each part of the retina transmits data via a corresponding set of fibers within the optic nerve. Damage to specific nerve fibers results in loss of the associated portion of the visual field.
Challenges with this test include its complexity, especially for older patients, and its subjective nature.
Changes in the visual field determine glaucoma severity. These changes indicate how much of the visual field is already damaged and which parts of the optic nerve are compromised. We call these ‘functional changes‘ as they directly impact visual function.
Fundus photo for Glaucoma detection
Alongside functional changes, Glaucoma causes visible structural changes in the optic nerve that can be observed during a fundus examination. The optic nerve begins at a point on the retina where all the nerve fibers gather, forming the optic disc (or optic nerve head). The nerve fibers are thickest near the optic disc, creating a depression or ‘hole’ within it. As Glaucoma progresses, this depression deepens due to increased pressure inside the eye. This pressure causes mechanical damage to the nerve fibers, leading to thinning and loss of function.
Another crucial area on the retina is the macula, which contains a high density of receptors responsible for image perception. While the entire retina senses images, the macula provides the sharpest, clearest vision. We use this area for tasks like reading, writing, and looking at fine details. Therefore, the damage to the macular area significantly impacts a patient’s visual quality and clarity. Nerve fibers carrying visual information from this crucial region are essential when evaluating the visual field. We prioritize assessing the macula’s health because it directly determines the quality of a patient’s central vision.
Unfortunately, even if the macula is healthy, damage to the nerve fibers transmitting its signals will still compromise vision.
Glaucoma OCT detection
The most effective way to get information about nerve states is OCT, which allows us to penetrate deep into the layers to see the nerve fiber layer separately, making it possible to assess the extent of damage and thinning to this layer in much more detail.
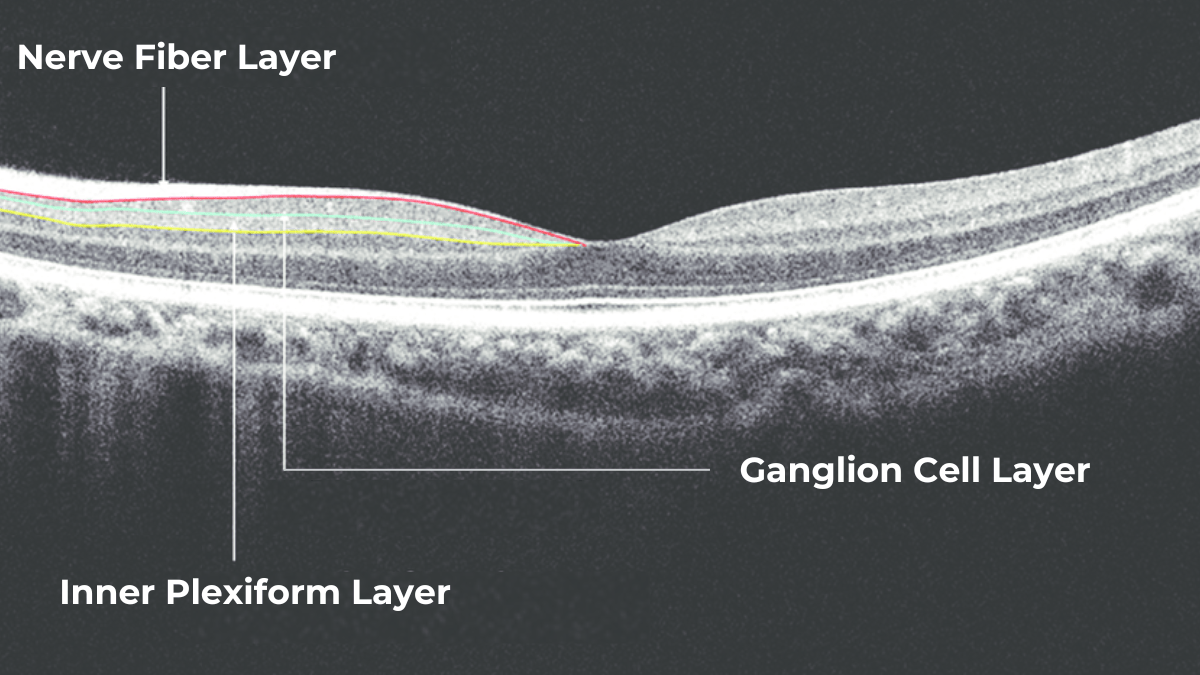
The Glaucoma OCT test provides valuable information about ganglion cells. These cells form the nerve fiber layer and consist of a nucleus and two processes. The short process collects information from other retinal layers, forming the inner plexiform layer. The ganglion cell layer comprises the cell nuclei, while the long processes extend out to create the nerve fiber layer.
Damage to the ganglion cells or their processes leads to thinning across these layers, which we can measure as the thickness of the ganglion cell complex. OCT often detects these microscopic changes before we can see them directly. This enables the detection of structural changes alongside the functional changes observed with standard visual field tests.
Ideally, OCT would be more widely accessible, as the human eye cannot detect early changes. However, how often a patient undergoes OCT depends on various factors. These include the doctor’s proficiency with the technology, the patient’s financial situation (as OCT can be expensive), and the overall clinical picture.
Ways to Enhance Early Glaucoma Detection
We surveyed eye care specialists, and there was a strong consensus that the most efficient ways to boost early glaucoma detection are regular eye check-ups (47%) and utilizing AI technology (40%). Educating patients was considered less significant (13%).

AI as a second opinion tool
AI offers valuable insights into glaucoma detection, analyzing changes that may not be visible to the naked eye or even on standard OCT imaging.
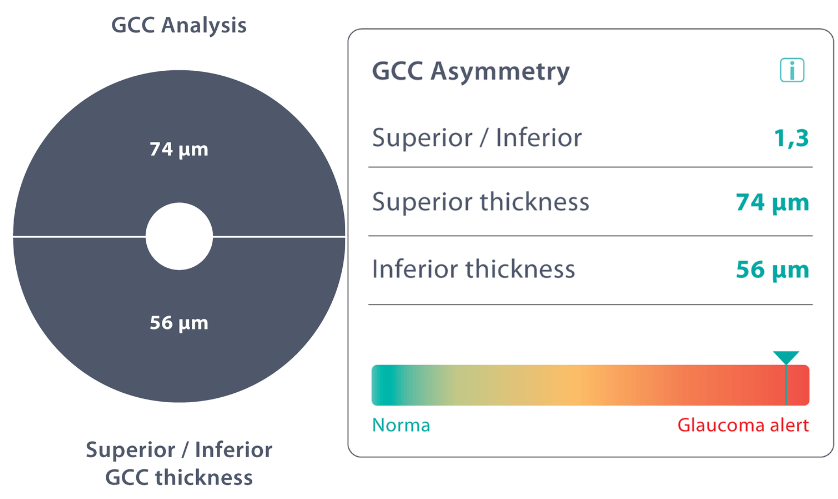
The Altris AI Early Glaucoma Risk Assessment Module specifically focuses on analyzing the OCT ganglion cell layer, measuring its thickness, and identifying any thinning or asymmetry. These measurements help determine a patient’s glaucoma risk. If the ganglion cell complex has an average thickness and is symmetrical throughout the macula, the module will assign a low probability of Glaucoma.

Asymmetries or variations in thickness increase the calculated risk, indicated by a yellow result color. Glaucoma GCC is often characterized by thinning or asymmetry, suggesting glaucomatous atrophy, indicating a high risk, and triggering a red result color.
Changes are labeled as ‘risk’ rather than a diagnosis, as other clinical factors contribute to a confirmed glaucoma diagnosis. Indicators of atrophy could also signal different optic nerve problems, such as those caused by inflammation, trauma, or even conditions within the brain.

It’s crucial to remember that AI ganglion cell layer OCT detection tools like this are assistive – they cannot independently make a diagnosis. Similarly, while helpful in assessing risk, they cannot completely rule out the possibility of developing a disease. This limitation stems from their reliance on a limited set of indicators. Like other technical devices, the module helps flag potential pathology but does not replace the clinician’s judgment.
AI can be incredibly valuable as a supplemental tool, especially during preventive exams or alongside other tests, to catch possible early signs of concern. However, medicine remains a field with inherent variability. While we strive for precise measurements, individual patients, not just statistical averages, must be considered.
Therefore, it is unrealistic to expect devices to provide definitive diagnoses without the context of a complete clinical picture.
Public Health Education
The asymptomatic nature of Glaucoma in its early stages, paired with limited public awareness, creates a fundamental barrier to early detection.
For example, 76% of Swiss survey respondents could not correctly describe Glaucoma or associate it with eye health.
A Canadian study similarly shows that less than a quarter of participants understand eye care professionals’ roles correctly and that most people are unaware eye diseases can be asymptomatic.
Crucially, these studies also found a strong desire across populations for more information about eye care, including Glaucoma (e.g., 97% of Swiss respondents agreed the public lacks knowledge, and 71% want more information). This indicates a receptive audience for targeted education initiatives.
Health education programs, like the USA EQUALITY study, demonstrate the potential to address this challenge. This study combined accessible eye care settings with a culturally sensitive eye health education program, targeting communities with high percentages of individuals at risk for Glaucoma.

Participants showed significant improvements in both glaucoma knowledge (a 62% increase in knowledge questions) and positive attitudes toward the importance of regular eye care (52% improvement).
These results show us that improving glaucoma detection involves more than medical tools. Successful education strategies should prioritize community outreach, partnering with community centers, primary care clinics, and local organizations to reach those lacking access or awareness of regular eye care.
Information about Glaucoma must be presented clearly and accessible, focusing on the basics—what Glaucoma is, its risk factors, and the importance of early detection. Addressing common misconceptions, such as the belief that Glaucoma can’t be present if vision is good, is crucial, as is targeting high-risk groups, including older adults, those with a family history of Glaucoma, and certain ethnicities.
Screening Programs and Regular visits
Community-based studies consistently demonstrate the benefits of targeted screening programs for early glaucoma detection in high-risk populations.
These programs are essential, as traditional glaucoma screening methods often miss individuals with undetected disease.

The USA Centers for Disease Control and Prevention (CDC) funded SIGHT studies focused on underserved communities, including those in urban areas with high poverty rates (MI-SIGHT, Michigan), residents of public housing and senior centers (NYC-SIGHT, New York), and the rural regions with limited access to specialist eye care (AL-SIGHT, Alabama). These programs successfully reached populations who often don’t have regular eye care.
Notably, the results across all three studies demonstrate the effectiveness of targeted programs – approximately 25% of participants screened positive for Glaucoma or suspected Glaucoma.
The SIGHT studies recognize that screening is just the first step, highlighting the importance of follow-up care, testing ways to improve follow-through, using strategies like personalized education, patient navigators, financial incentives, and providing free eyeglasses when needed.
Summing up

FDA-cleared AI-powered OCT Glaucoma Risk Assessment
Glaucoma’s insidious nature demands better early detection strategies. While existing methods are essential, we must also invest in new technologies like AI, enhance public health education about Glaucoma, and focus on targeted screening within at-risk populations. Combining these approaches can protect sight and reduce the burden of glaucoma-related blindness.
