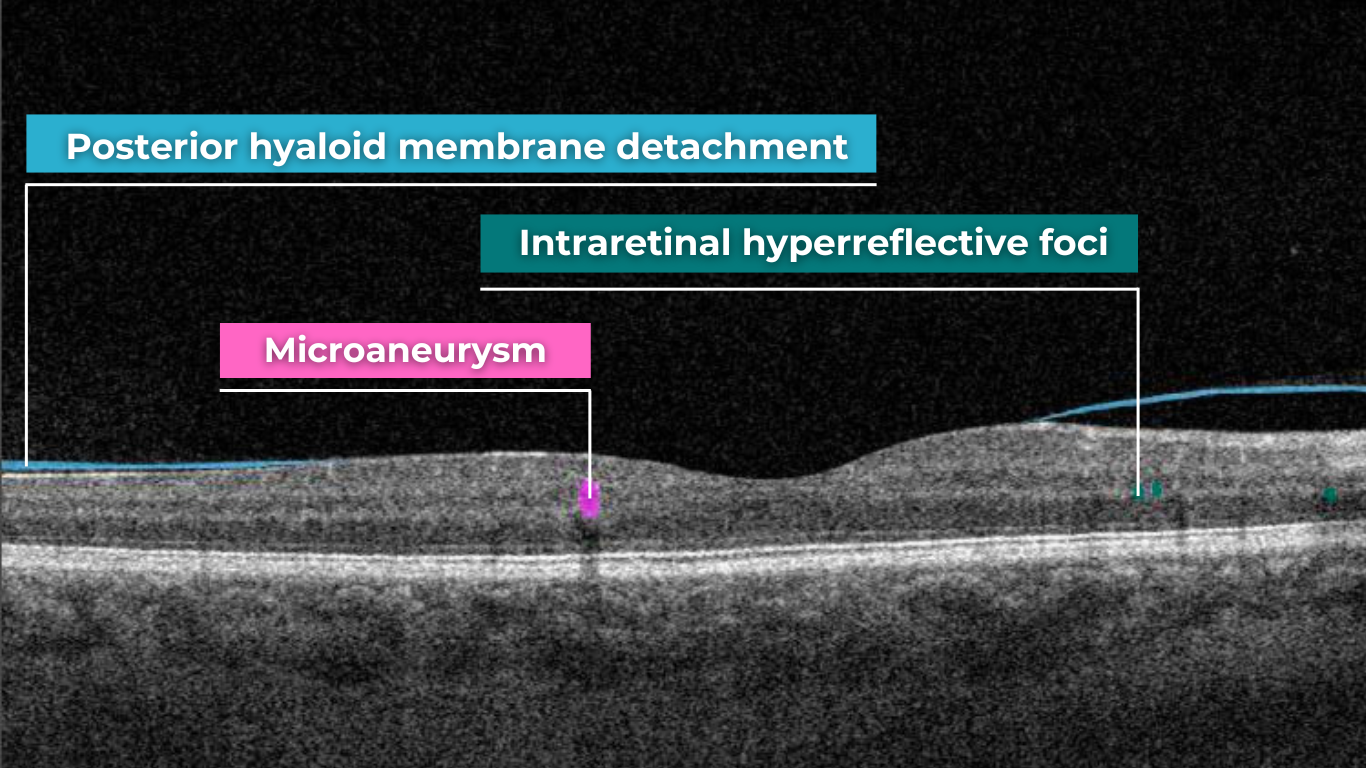
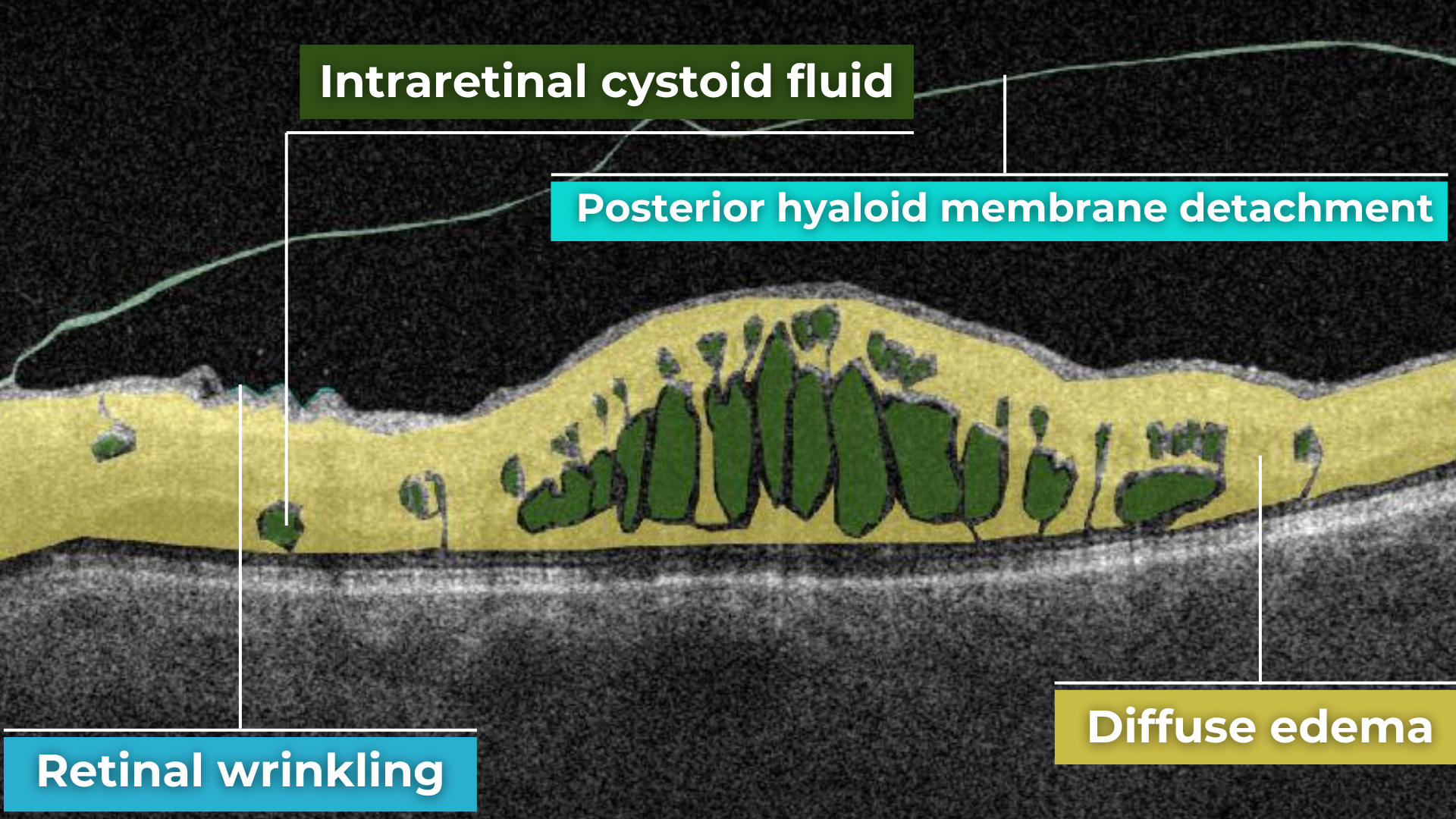
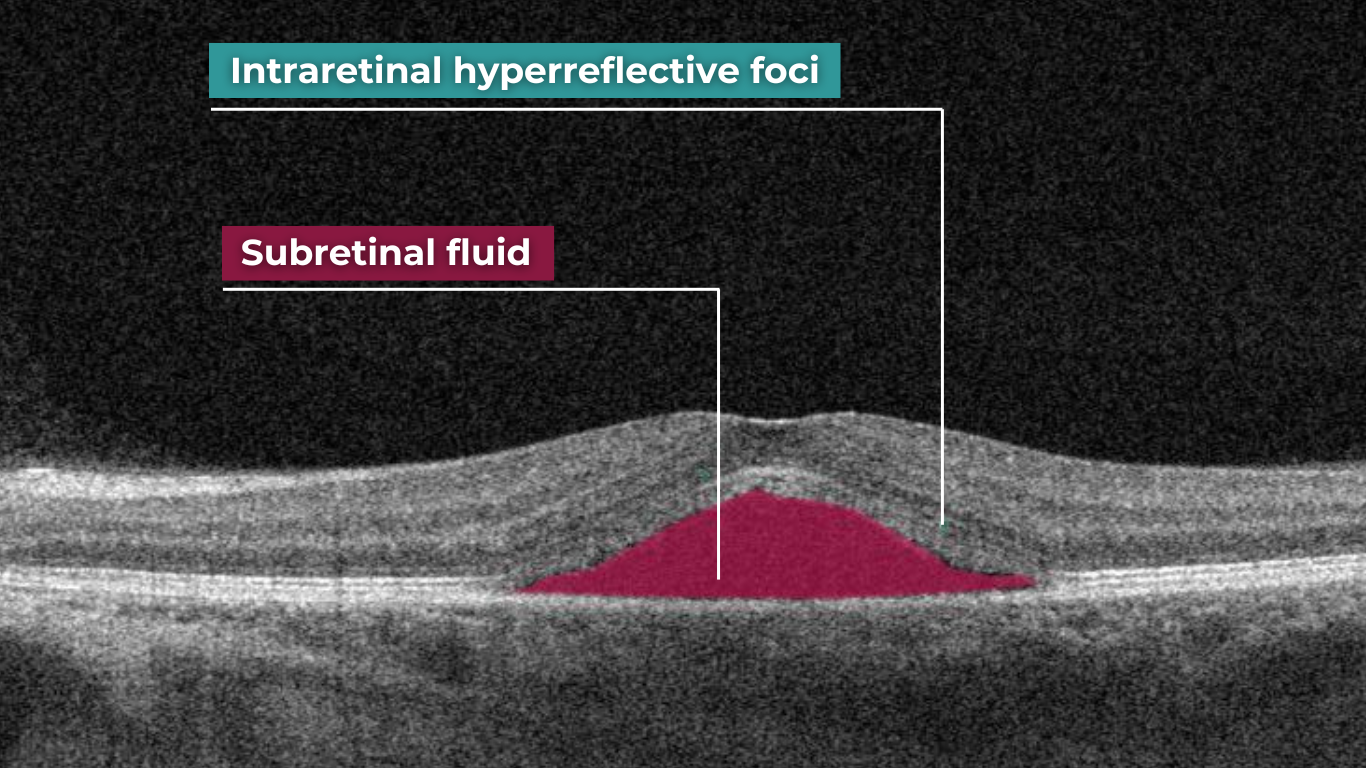
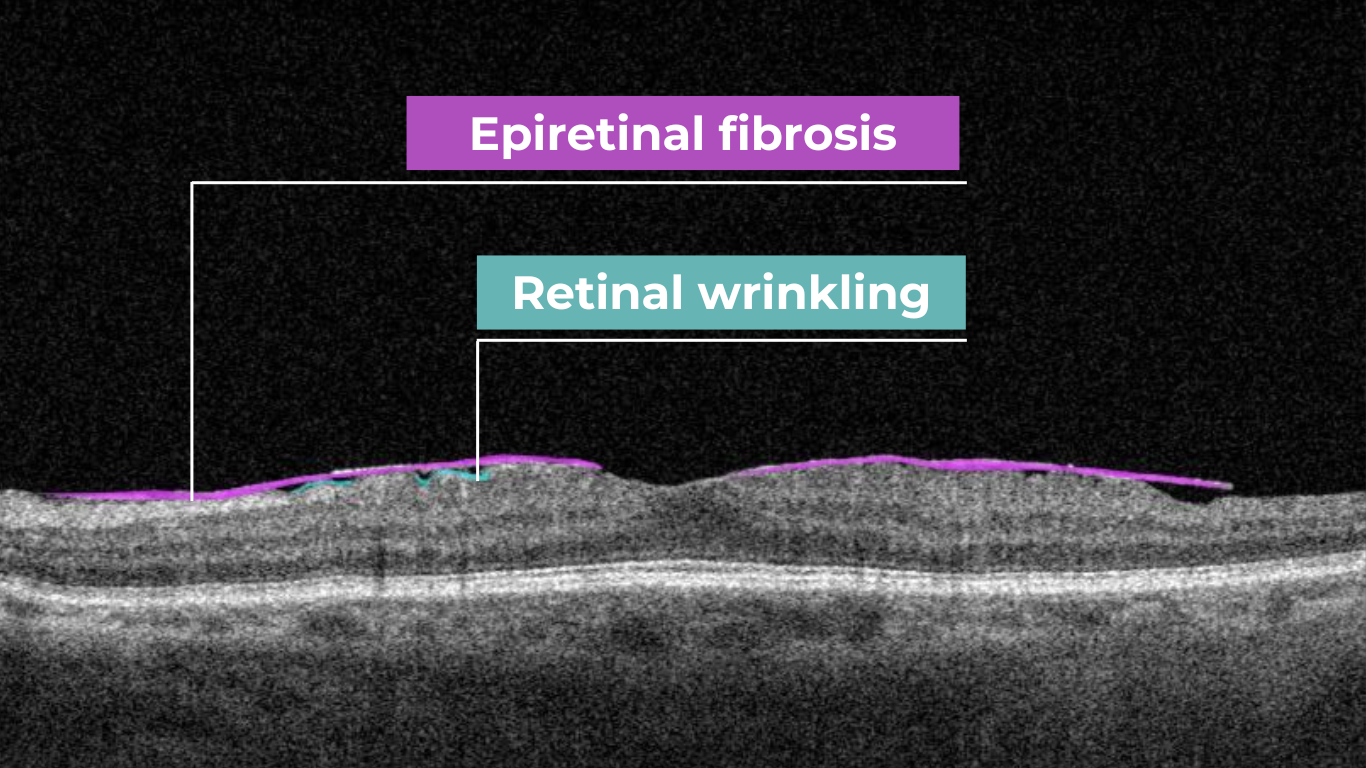
Recently Posted
-

Business Case: Altris AI for Jeff Sciberras Optometry
 Altris Inc.
10.07.20231 min read
Altris Inc.
10.07.20231 min readBusiness Case: Altris AI for Jeff Sciberras Optometry
The Client: Canadian Optometry Clinic
Jeff Sciberras Optometry Clinic is an established eye care facility in Mississauga, Canada. They have been recognized as the Top Choice Optometry Clinic for the past five years running in this large Canadian city.
Dr. Jeff Sciberras is proud of his high patient satisfaction rate: 92% of those surveyed would refer a friend, colleague, or family member to this establishment.
Dr. Sciberras aims to provide comprehensive eye care, with a desire to utilize leading technologies and the delivery of premium eye care products.
Recent technology investments include OCT, which allows earlier diagnosis and greater in-house management capabilities.

AI for OCT Analysis
Make your eye care business technological
The Challenge: The optometry clinic has just purchased a brand new Optopol Revo OCT equipment and the support was needed in OCT scan interpretation. OCT is one of the most accurate methods of retina diagnostics however, the interpretation of OCT scans can be challenging and time-consuming, for both doctor and patient.
The Result:
Dr. Sciberras has been extremely satisfied with the support that the Altris AI platform provides:
- Increased confidence when working with the new OCT device · more profound analysis of OCT scans
- More adequate referral of complex cases.
- Scan summaries for the patient.
- Earning patient confidence and trust: The image of the innovative optometry center is enhanced to their patients and families.
- The AI Segmentation/Classification Module is invaluable for the optometry center as this module helps in the identification of 70+ pathologies and pathological signs.
The introduction of OCT with Altris AI has transformed my practice literally overnight. The integration was seamless and Altris customer support has been outstanding.
Overall, Dr. Sciberras has been impressed with the experience and support Altris AI provides and is happy to have chosen to partner with them for his leading eye care center.
-

DICOM Format: Benefits of Managing DICOM images
 Mark Braddon
31.05.20236 min read
Mark Braddon
31.05.20236 min readDICOM Format: Benefits of Managing DICOM images
DICOM file format (Digital Imaging and Communications in Medicine) was developed by the American College of Radiology (ACR) and the National Electrical Manufacturers Association (NEMA) as a standard for exchanging medical images and related information across different healthcare systems. It serves as a universal language for medical imaging, enabling interoperability between various imaging devices and systems. DICOM ensures that medical images can be exchanged and viewed consistently regardless of the manufacturer or modality.
DICOM image format supports a broad range of medical imaging modalities, including X-ray, MRI, OCT, ultrasound, nuclear medicine, and more. It also covers related data, such as patient information, study details, image annotations, and results.

AI for OCT analysis
Test how it works yourself
As the DICOM format continues to evolve to keep up with advancements in medical imaging technology, our article aims to raise awareness among ophthalmologists and optometrists about the DICOM file format.
What is DICOM format? You can also watch a short video about DICOM and non-DICOM file formats.
What is DICOM file format?
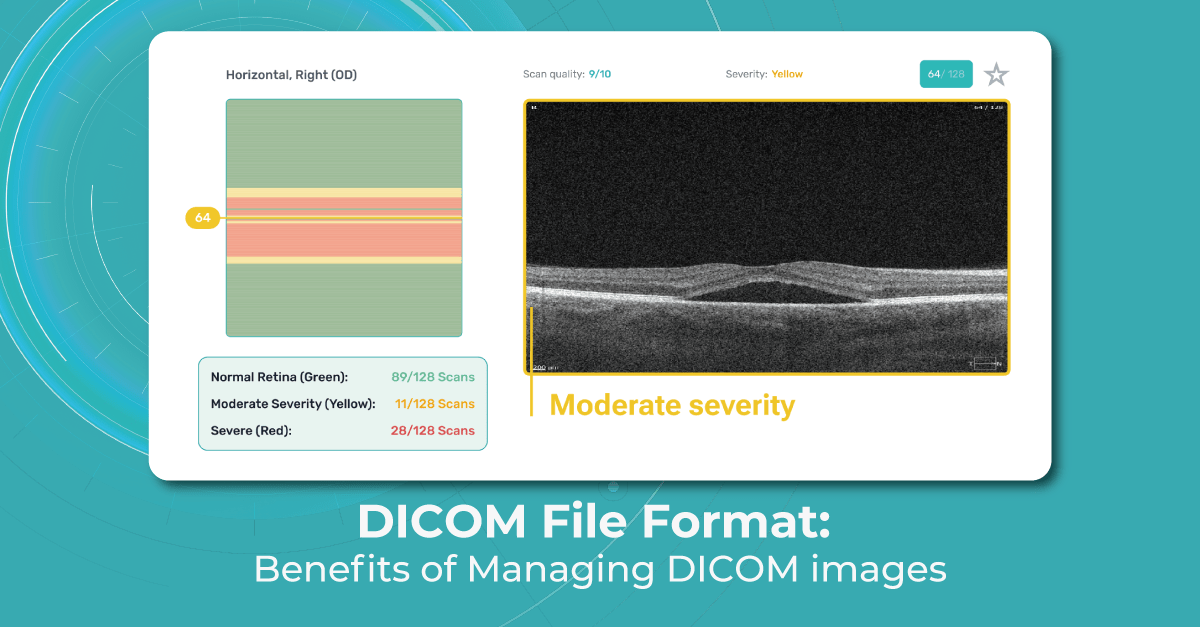
Image files that adhere to part 10 of the DICOM standard are commonly known as “DICOM format files” or simply “DICOM files,” and their file extension is “.dcm.” In ophthalmology, DICOM is a widely used file format for storing and transmitting medical images. DICOM files are used to store various types of ophthalmic images as well, including retinal images, optical coherence tomography (OCT) scans, visual field tests, and angiography images.
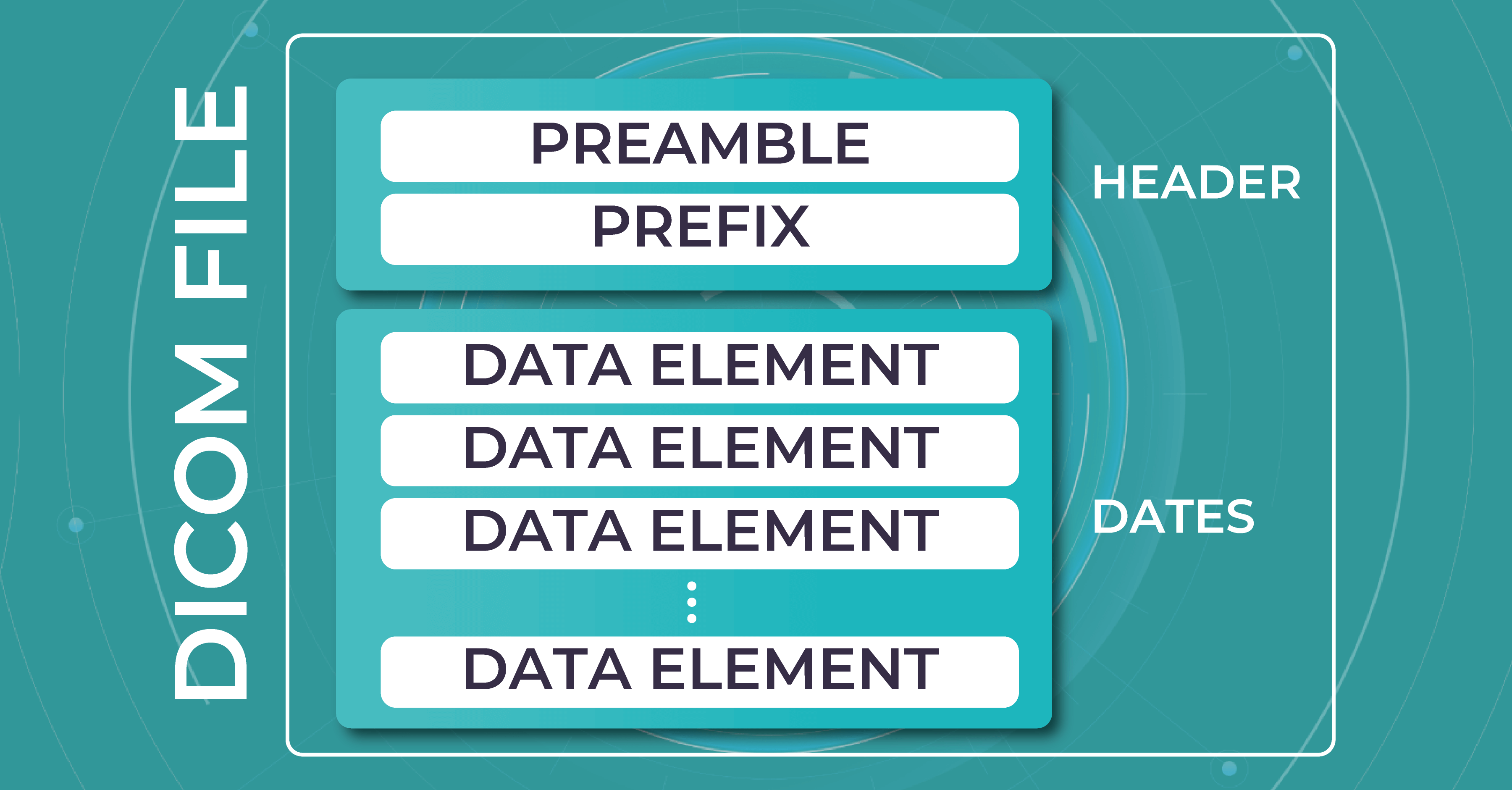
DICOM files consist of two main components: the header and the image data. The header contains metadata that describes the patient, study, series, and image acquisition parameters.

This metadata includes information such as patient demographics, image acquisition parameters (e.g., imaging modality, image orientation, pixel spacing), and any annotations or measurements made on the image. The image data itself is typically stored in a compressed format, such as JPEG or JPEG 2000, within the DICOM file.
DICOM files also support the exchange of images and associated data between different medical imaging devices and systems. This enables eye care specialists to easily share and access ophthalmic images across different platforms, such as picture archiving and communication systems (PACS), ophthalmic imaging devices, and electronic health record (EHR) systems.
By using DICOM, ophthalmologists and optometrists can efficiently store, retrieve, and analyze ophthalmic images, ensuring accurate diagnoses and effective patient care. In the next paragraphs, we will tell you more about the benefits of the DICOM file format for eye care specialists.
Benefits of DICOM format
The DICOM standard ensures interoperability between different vendors’ OCT devices and facilitates seamless data sharing and analysis. The main difference between DICOM and other image formats is that it groups information into data sets. A DICOM file consists of several tags, all packed into a single file. It stores such info as:
- demographic details about the patient
- imaging study’s acquisition parameters
- image dimensions
- matrix size
- color space
- an array of additional non-intensity information necessary for accurate image display by computers.
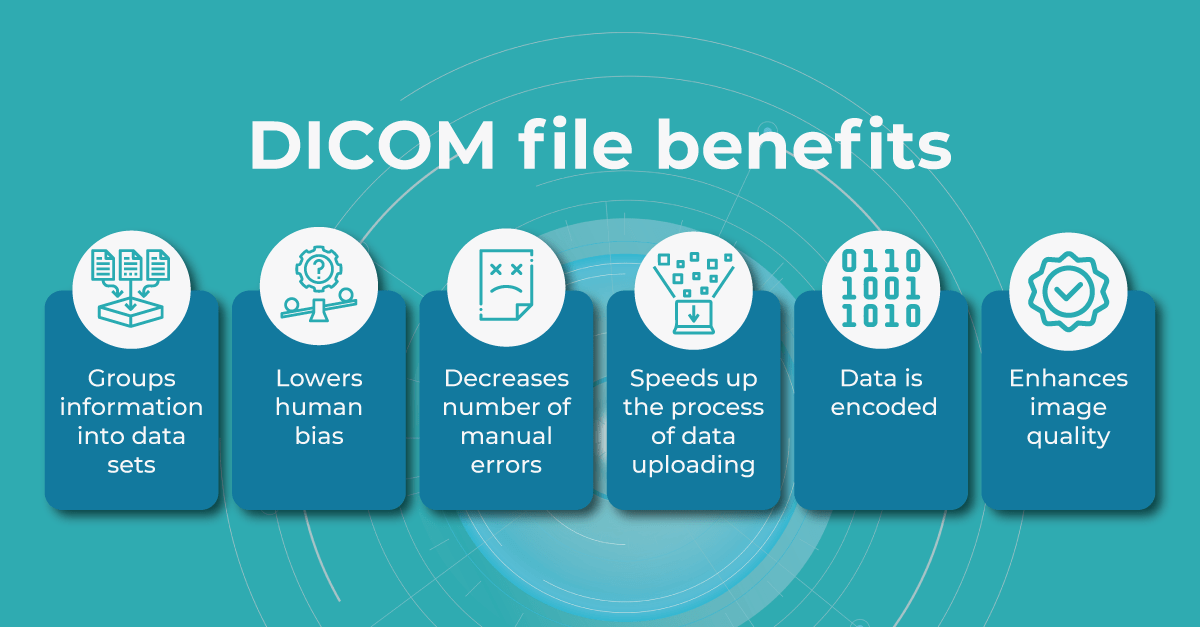
If you have to enter the patient’s information manually, there’s always a chance you can misspell the name or other information. However, when using a DICOM file to store patients’ information and monitor patients’ health, eye care specialists can be sure the chance of human bias is much lower.
When you work in an optometry practice or a clinic, you may spend a lot of time filling in the details every time you upload a file. And if your clinic is busy and you do 30-50 uploads daily, it could take hours. Using DICOM image format significantly speeds up the process and reduces errors.

Another benefit of the DICOM image format is that the header data information is encoded within the file so that it cannot be accidentally separated from the image data.
DICOM files can be stored in a DICOM server or transmitted between DICOM-compliant systems using the DICOM network protocol (DICOM C-STORE or DICOMweb). DICOM SR (structure reporting) allows for the structured representation of measurement data and annotations in OCT images. It enables the storage of quantitative measurements, such as retinal thickness or optic nerve parameters, as structured data within the DICOM file.
In addition, eye care specialists are able to manipulate the brightness of the image when using the DICOM viewing software. Some areas of an image can be increased or decreased for a better viewing and diagnostic experience.
Is DICOM file format popular among OCT providers?
When it comes to optical coherence tomography, many OCT device manufacturers and software providers support the DICOM standard for storing and exchanging OCT images. Some of the prominent OCT providers that offer DICOM support include:
- Heidelberg Engineering is a well-known provider of OCT devices and software solutions for ophthalmology. They offer OCT devices like the Spectralis OCT, which supports DICOM connectivity. The DICOM capabilities of their systems enable seamless integration with PACS and other healthcare systems.
- Carl Zeiss Meditec is a leading manufacturer of ophthalmic devices, including OCT systems. Their OCT devices, such as the Cirrus OCT, are DICOM-compatible, allowing for efficient storage and sharing of OCT images with other DICOM-compliant systems.
- Topcon Medical Systems is another prominent provider of OCT devices. Their OCT systems, such as the Topcon 3D OCT, support DICOM connectivity, enabling interoperability with other DICOM-enabled devices and systems.
- NIDEK offers a range of ophthalmic imaging devices, including OCT systems. Their OCT platforms, such as the NIDEK RS-3000, support DICOM, allowing for seamless integration with DICOM-compliant infrastructure, such as PACS and EHR systems.

AI for OCT analysis
Test how it works yourself
These are just a few examples of OCT providers that support the DICOM standard. It’s important to note that DICOM support may vary among different models and versions of OCT devices from each manufacturer. We recommend you consult with the specific manufacturer or review their product documentation to confirm the DICOM capabilities of their OCT systems.
Why do we recommend using DICOM file format with Altris AI?
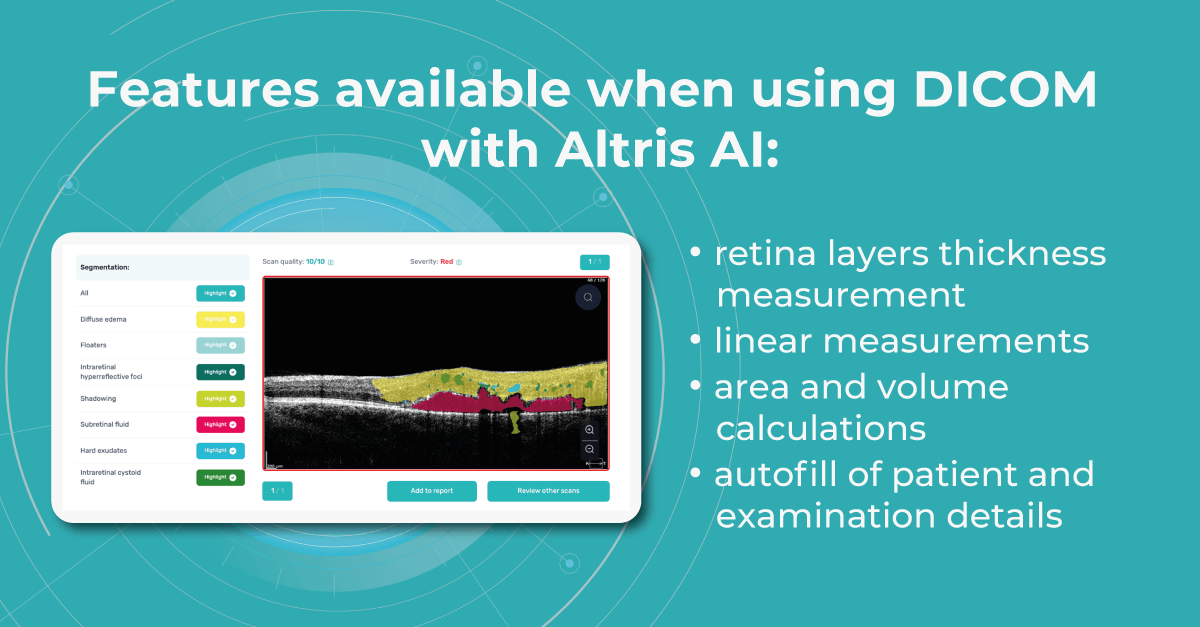
Modern DICOM viewer software extends beyond simple viewing. It can enhance image quality, generate additional data, take measurements, and more, and Altris AI is no exception. Using the DICOM image file gives you more opportunities within the platform.
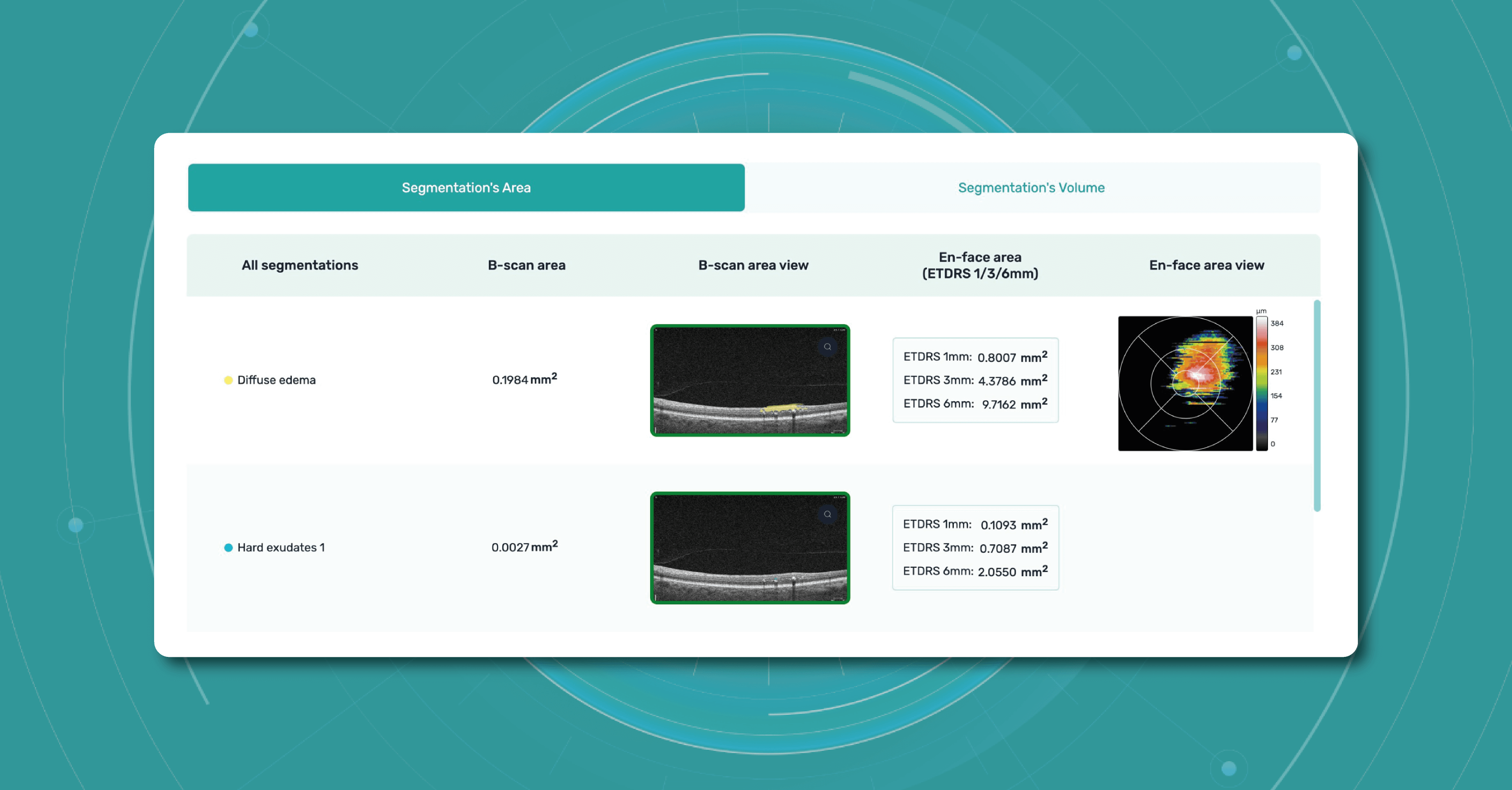
Such features as
- retina layers thickness and linear measurements

- area and volume calculations

are only available when using the DICOM file format. This is because it contains the original image pixel data without modifying the study metadata. In case you upload an image, retina layers thickness won’t be available, as well as the measurements.

AI for OCT analysis
Test how it works yourself
Another advantage of the DICOM format is that you can add patient and examination details in a few clicks by just uploading a DICOM file since this information is being pulled out automatically.

In the case of other image formats, when uploading an examination, you would have to manually fill in a bunch of information such as scan widths, eye type, etc.
Considering all mentioned above, using DICOM format files saves time, increases efficiency, and gives you more opportunities within the Altris AI platform.
Summing up
What is DICOM format? In conclusion, the DICOM file format proves to be a valuable asset for eye care specialists. Its unique characteristics, such as grouping information into data sets and incorporating standardized tags within a single file, offer many advantages.
This format ensures the preservation of accurate and comprehensive data, reducing the potential for human error and minimizing the risk of data loss or misinterpretation. The DICOM file format streamlines the archival, organization, and display of images, optimizing the workflow of eye care specialists.
By adhering to the DICOM standard, OCT devices and software solutions ensure compatibility, interoperability, and consistent data representation across different platforms. This enables efficient communication and collaboration among healthcare professionals, enhances research capabilities, and promotes the broader use and exchange of OCT imaging data.

AI for OCT analysis
Test how it works yourself
With its widespread adoption and compatibility with various medical imaging systems, DICOM empowers ophthalmologists and optometrists to provide efficient and high-quality care while promoting seamless collaboration and knowledge sharing within the field. Ultimately, the DICOM file format plays a vital role in enhancing patient care, advancing research, and fostering innovation in the field of eye care.
-

How 7 Leading Optometry Centers Provide Innovations in Eye Care
 Maria Martynova
08.05.20239 min read
Maria Martynova
08.05.20239 min readTop modern optometry centers are not afraid of embracing effective eye care innovation. Some offer home eye tests, others create mobile apps to try on frames remotely. There are optometry centers that use artificial intelligence to empower optometrists in OCT/ fundus interpretation. We’ve collected 7 optometry centers that are using technology now to win the competition.
From advanced diagnostic and treatment technologies to personalized care and patient education, these centers are transforming the way clients approach and bring innovations in eye care.

AI for OCT
FDA-cleared system for your business
Optometry meets technology: AI, AR, mobile apps, and home eye tests
Augmented Reality (AR), mobile apps, and home eye tests are emerging trends that are changing the way people receive eye care.
- AR technology uses the camera lens on a mobile device or your PC as the method to deliver information and graphics. A user accesses an AR application, and the camera viewpoint incorporates the data directly into the perspective in real time. With AR apps for eyewear and exams, anyone can have a large selection of glasses and other services from their homes, offices, or on the go.
- Mobile apps offer a wide range of eye care services, from information on eye health and tips for maintaining healthy vision to virtual vision screenings. Moreover, mobile apps are also used to educate both young and experienced optometrists. We strongly believe that educational mobile apps inevitably become an additional efficient tool for OCT education because they are accessible and interactive.
- Another one of the innovations in eye care is Home eye tests are also often enabled by digital vision testing tools. They are becoming more and more common and offer a convenient and cost-effective way to monitor vision changes.
- As for AI use in optometry practice, it allows its users to see a broader perspective of a patient’s eye health. Incorporating AI streamlines billing procedures, expands the input of electronic health records (EHRs), optimizes claims management, and improves cash flow. AI technology can also be used in cooperation with AR assisting in the glasses selection.
Although these innovations in optometry and ophthalmology provide more comprehensive access to eye care and improve patient engagement, many optometry practices are still hesitating to add such innovations to their routine. That is why we prepared the info about 7 famous optometry practices that are already using innovations in eye care.
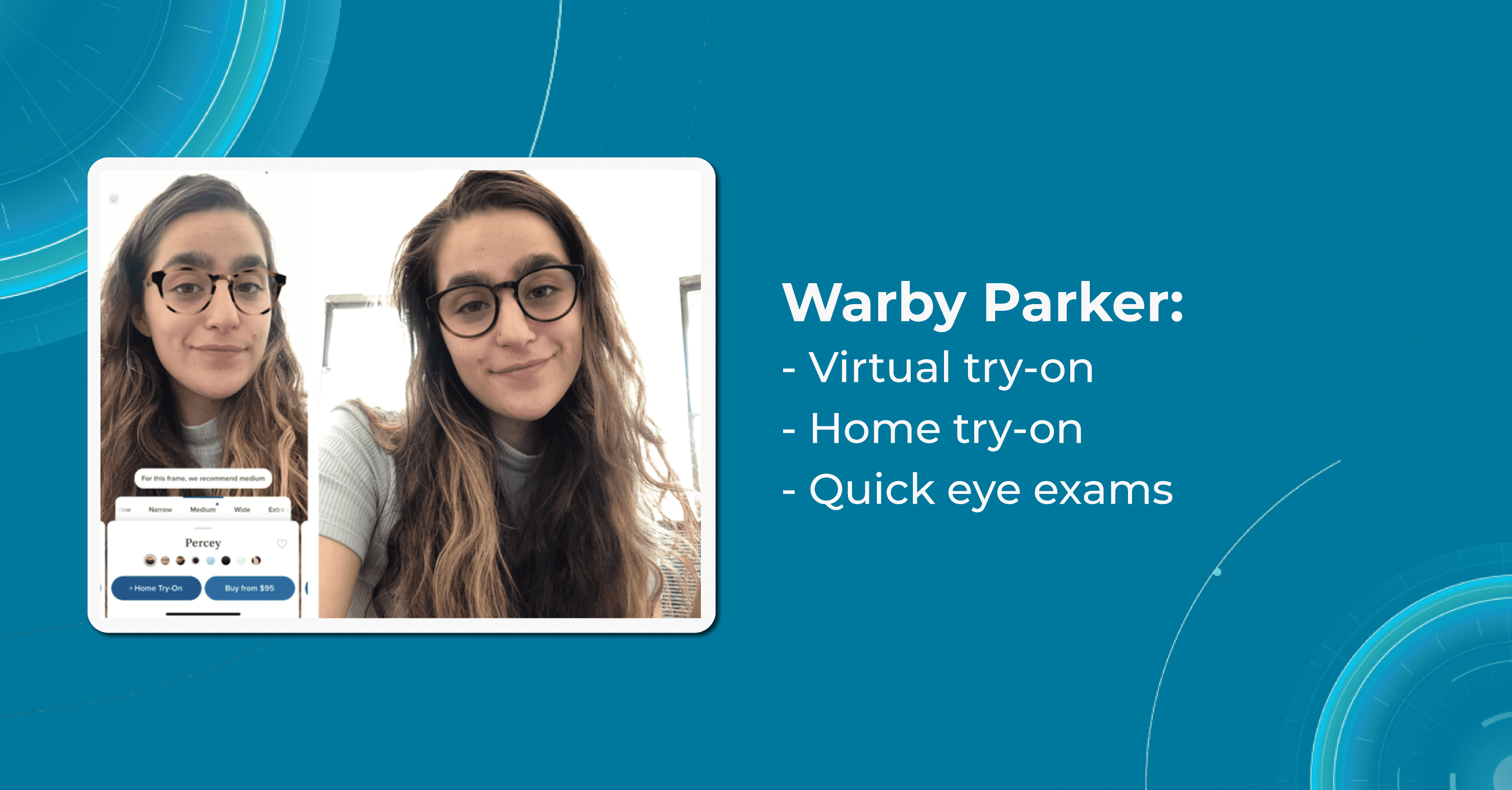
Warby Parker

Warby Parker started its way in 2010 when the founders of the company were students. One of them lost his glasses during a tourist trip. The cost of replacing them was so high that he spent his first semester of graduate school without them. That is why the company’s mission is to provide affordable, high-quality eyewear to consumers, while also addressing the issue of access to vision care.
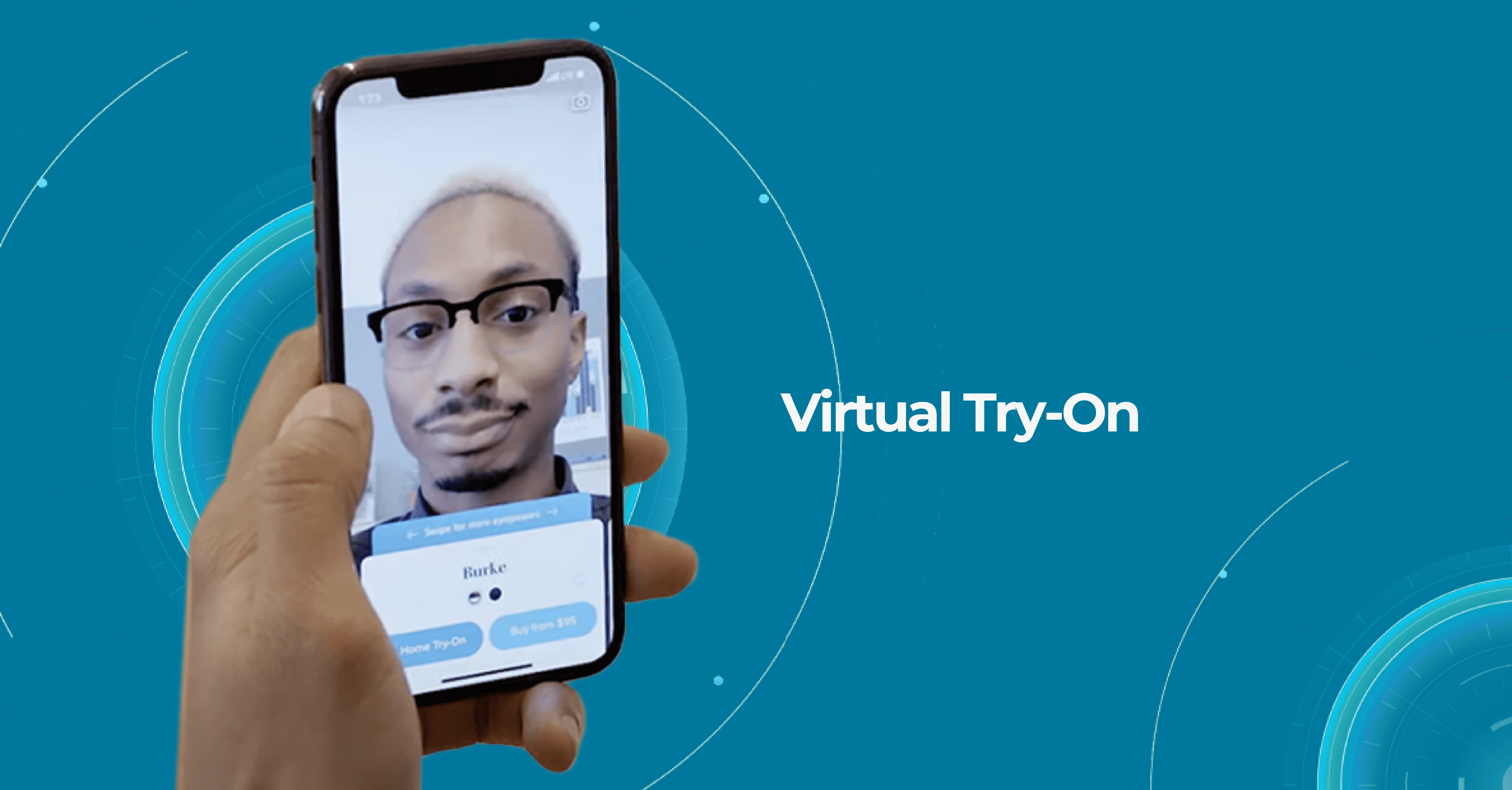
One of Warby Parker’s unique innovations in eye care is its Home Virtual Try-On program, which allows customers to try on up to five frames at home for free before making a purchase. This program makes it easier for customers to find the perfect pair of glasses and eliminates the need for them to go to a physical store to try on frames.

Warby Parker also offers an online eye exam called the Virtual Vision Test. It is designed to provide customers with a convenient and affordable way to obtain a prescription for glasses or contacts from the comfort of their own homes.
The Virtual Vision Test is a telemedicine service that uses technology to allow customers to take an eye exam using their computer or smartphone. The test is not meant to replace a comprehensive eye exam performed by an eye doctor, but rather to provide a convenient option for those who need a prescription renewal or have mild refractive errors.
After completing the test, the results are reviewed by a licensed ophthalmologist or optometrist, who will issue a prescription if appropriate. The customer can then use the prescription to purchase glasses or contacts from Warby Parker or any other provider.
Lenskart

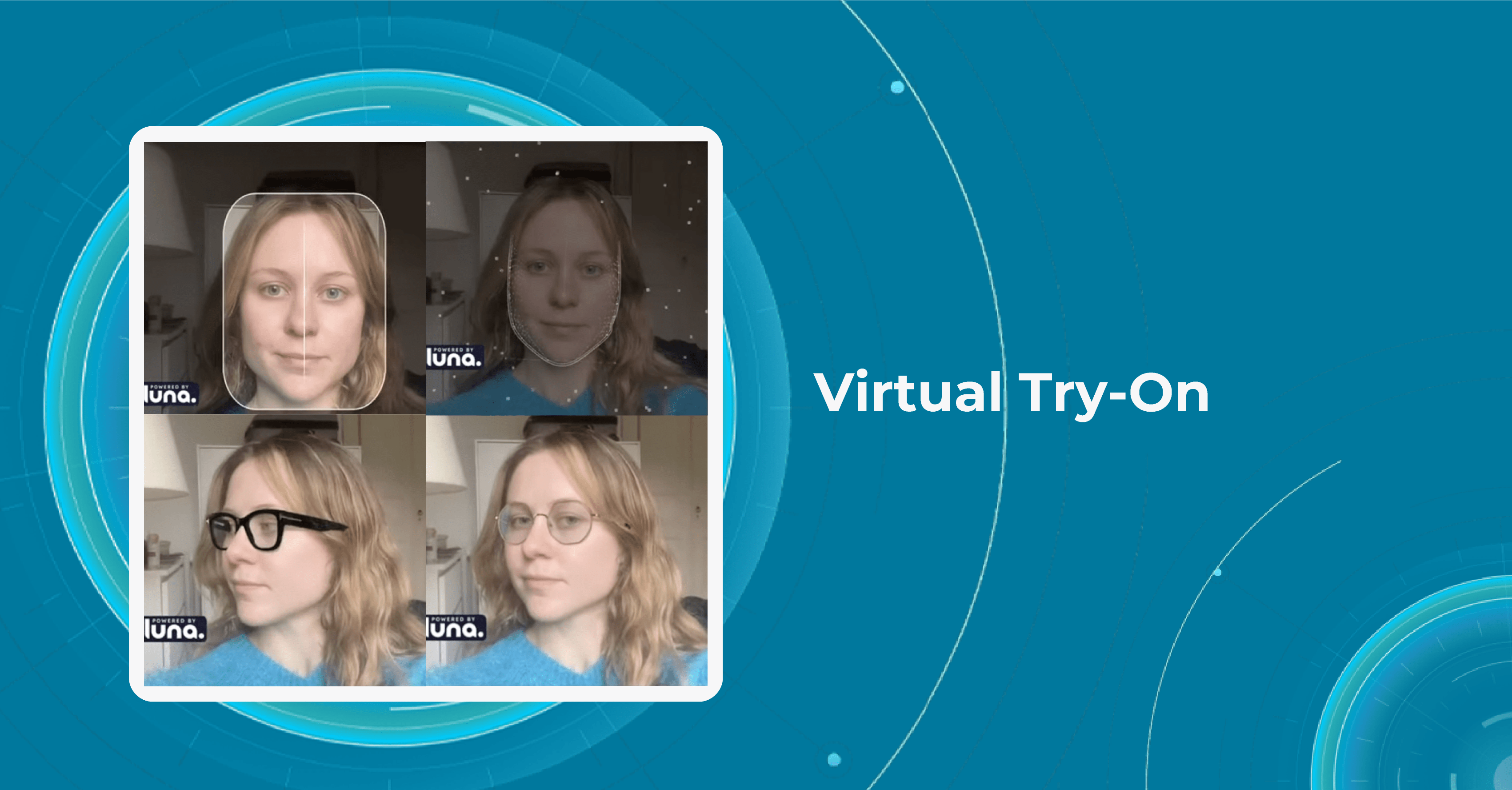
Lenskart is a fast-growing company of innovations in eye care in India focused on making eyewear more affordable for everyone. To achieve this goal, the company has developed a number of innovative technologies and business models, including a mobile app that allows customers to try on frames virtually and a home vision testing service that allows to check their prescriptions from the comfort of their own home.
One special feature of the Lenskart app is the “3D Try-On” feature, which uses 3D imaging technology to create a model of the customer’s face and allows them to try on different frames virtually. This feature helps get a better sense of how a particular frame will look on a customer’s face before making a purchase.

Another one of Lenskart’s innovations in eye care is the Home eye test, designed to provide people with a convenient and affordable way to obtain a prescription for glasses or contact lenses. To take the Lenskart Home Eye Test, customers must first book an appointment on the company’s website or mobile app.
The eye test includes a visual acuity test, a color vision test, and a refractive error test. The optometrist will also check the customer’s eye health and recommend any necessary follow-up exams or treatments. After the test, the optometrist will provide a prescription, which the customer can use to purchase glasses or contacts from Lenskart or any other provider.
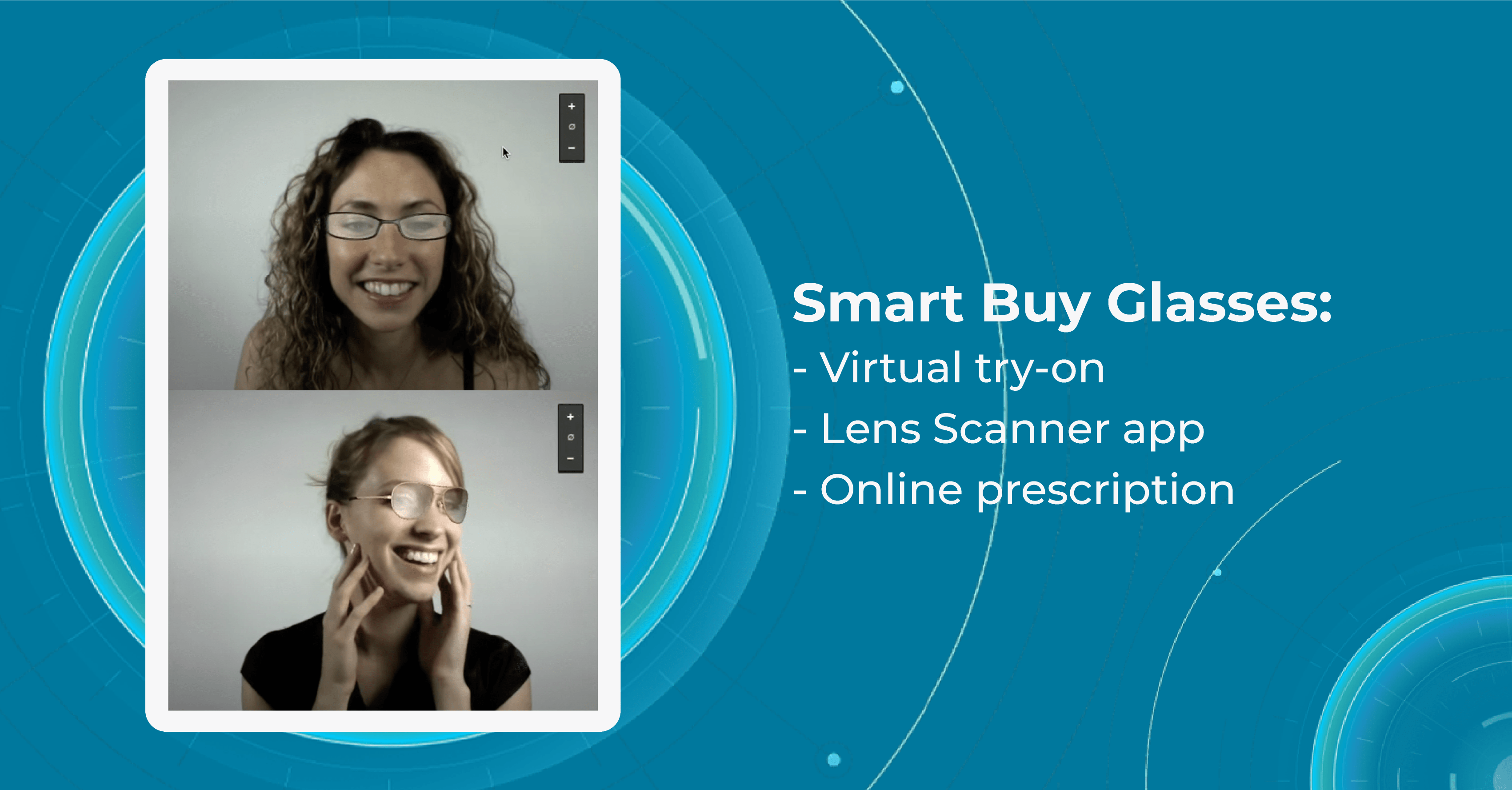
SmartBuyGlasses

SmartBuyGlasses is an online eyewear retailer that was founded in 2006. The company is headquartered in Hong Kong, but it operates in more than 20 countries worldwide. Company’s Virtual Try-On feature is available on the website and allows customers to upload a photo of themselves and try on glasses virtually using augmented reality.
After the website generates a 3D model of the customer’s face, they can adjust the position and size of the glasses to get a better sense of how they will look on their faces. The virtual try-on innovations in eye care also allow to share images of themselves wearing the glasses with their friends and family to get feedback on which pair looks best on them.

Another eye care innovation of SmartBuyGlasses is a Lens scanner app that uses advanced technology to scan the user’s current eyeglasses lenses and analyze the prescription, allowing to order a new pair of glasses online without visiting an eye doctor.
The app works by instructing the user to place their current eyeglasses on a flat surface and position their smartphone camera above the lenses. The app then captures a series of images and uses advanced algorithms to analyze the curvature, thickness, and other factors of the lenses to determine the prescription.
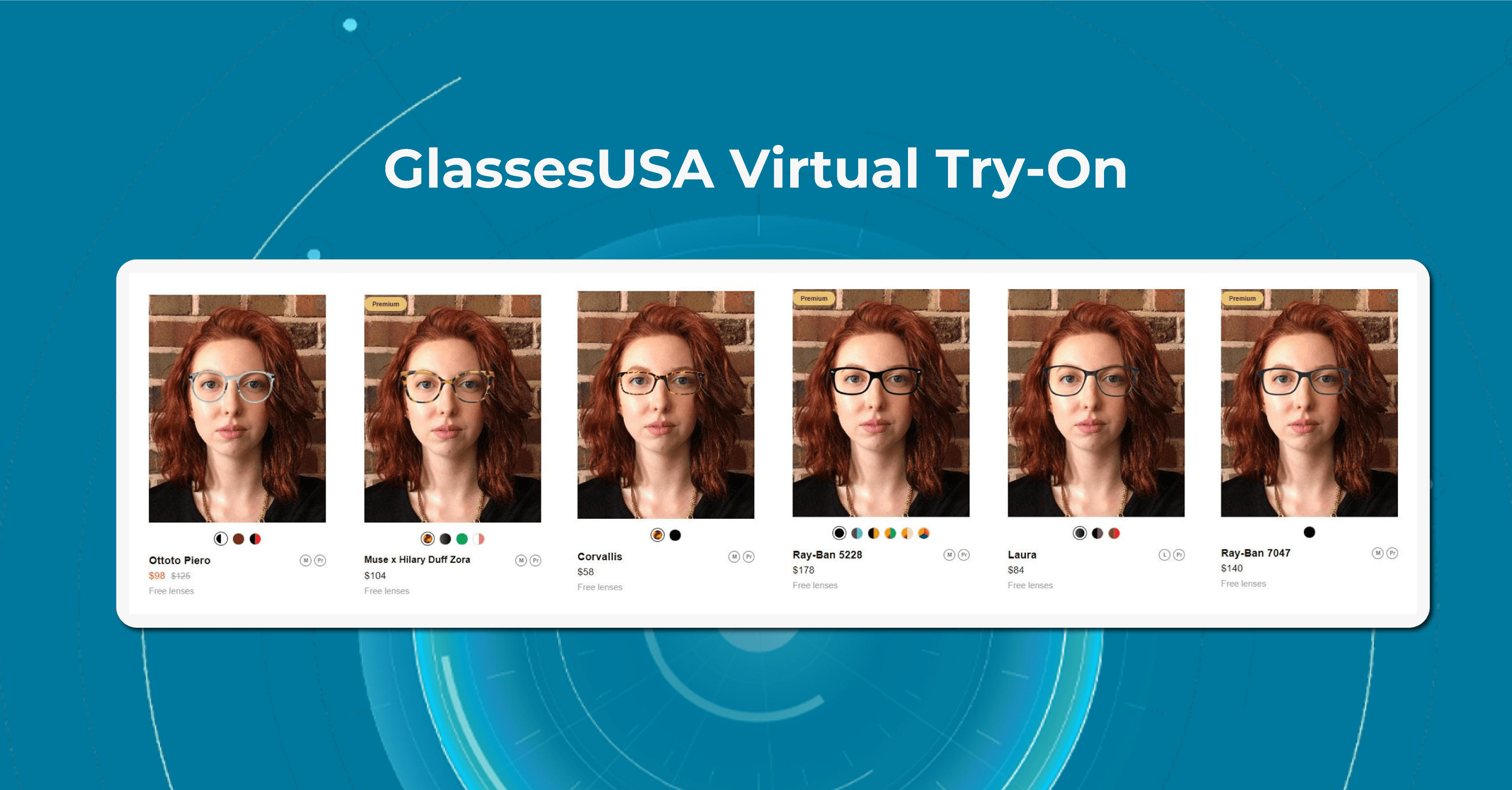
GlassesUSA

GlassesUSA is an innovative and socially responsible eyewear retailer that is committed to providing quality products and services to its customers. With its focus on technology, sustainability, and social impact, GlassesUSA has become a popular choice for customers in the United States and around the world.
One of the innovations in eye care of GlassesUSA that is worth paying attention to is a Prescription Scanner app. The app works by guiding the user through a series of steps to scan their face and eyes using their smartphone camera. It uses advanced algorithms to analyze the user’s facial features and measure the distance between their pupils, which is a crucial factor in determining the correct prescription for eyeglasses.

Once the scanning process is complete, the GlassesUSA app provides the user with their personalized prescription and recommendations. The app also offers a Virtual Try-On feature that allows users to see how different frames will look on their faces before making a purchase.
Another feature is a Find-your-Frame Quiz on the website. The quiz consists of a series of questions that ask users about their face shape, personal style, and preferences for eyeglass frames, such as color, material, and shape. Based on the user’s responses, the specially designed program generates a personalized selection of eyeglasses frames that are recommended for their face shape and style preferences.
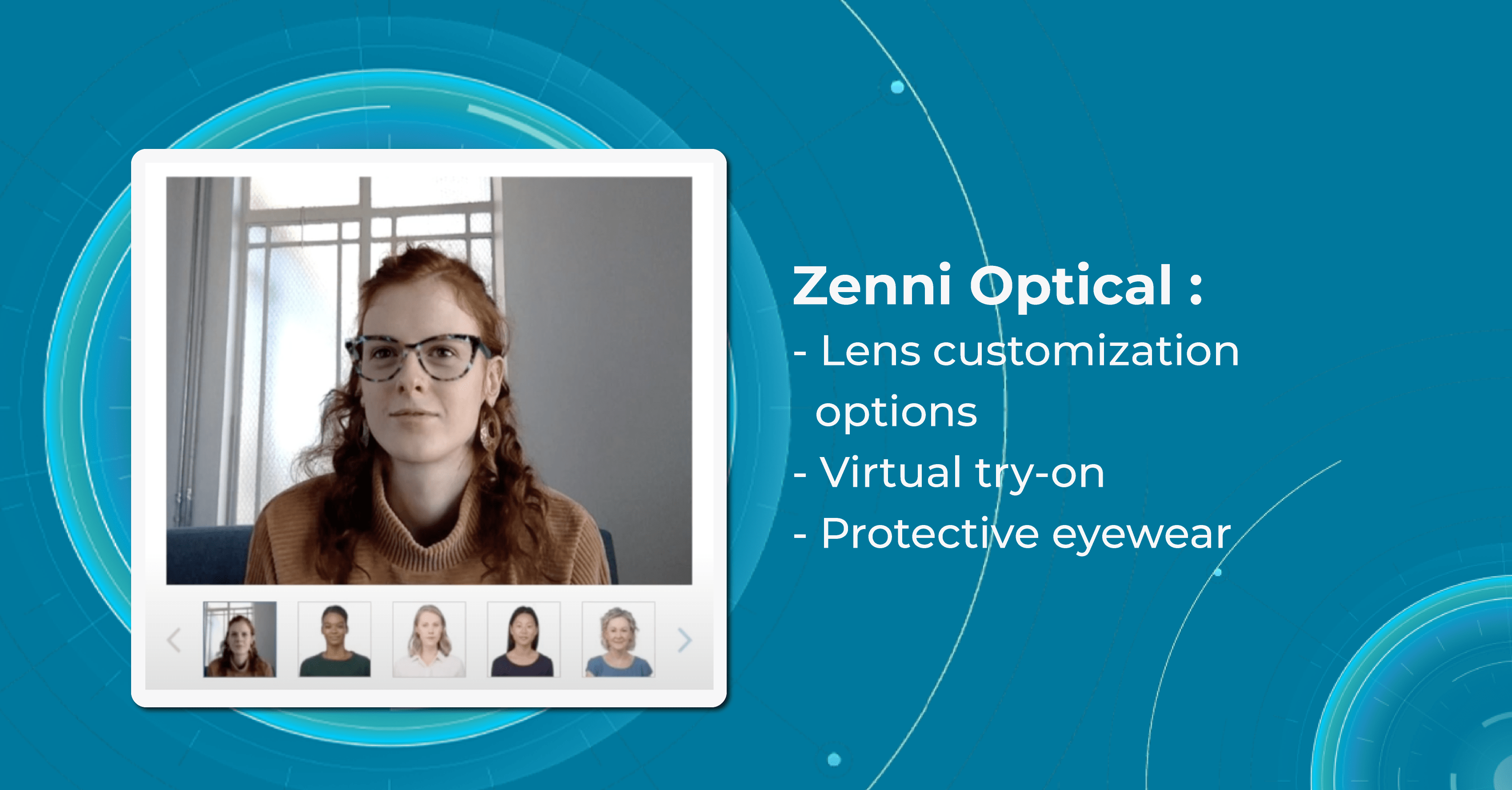
Zenni Optical

Zenni Optical offers a wide range of eyewear products, including prescription glasses, sunglasses, and sports eyewear. The company offers glasses at significantly lower prices than traditional brick-and-mortar stores, which has made it a popular choice for customers.
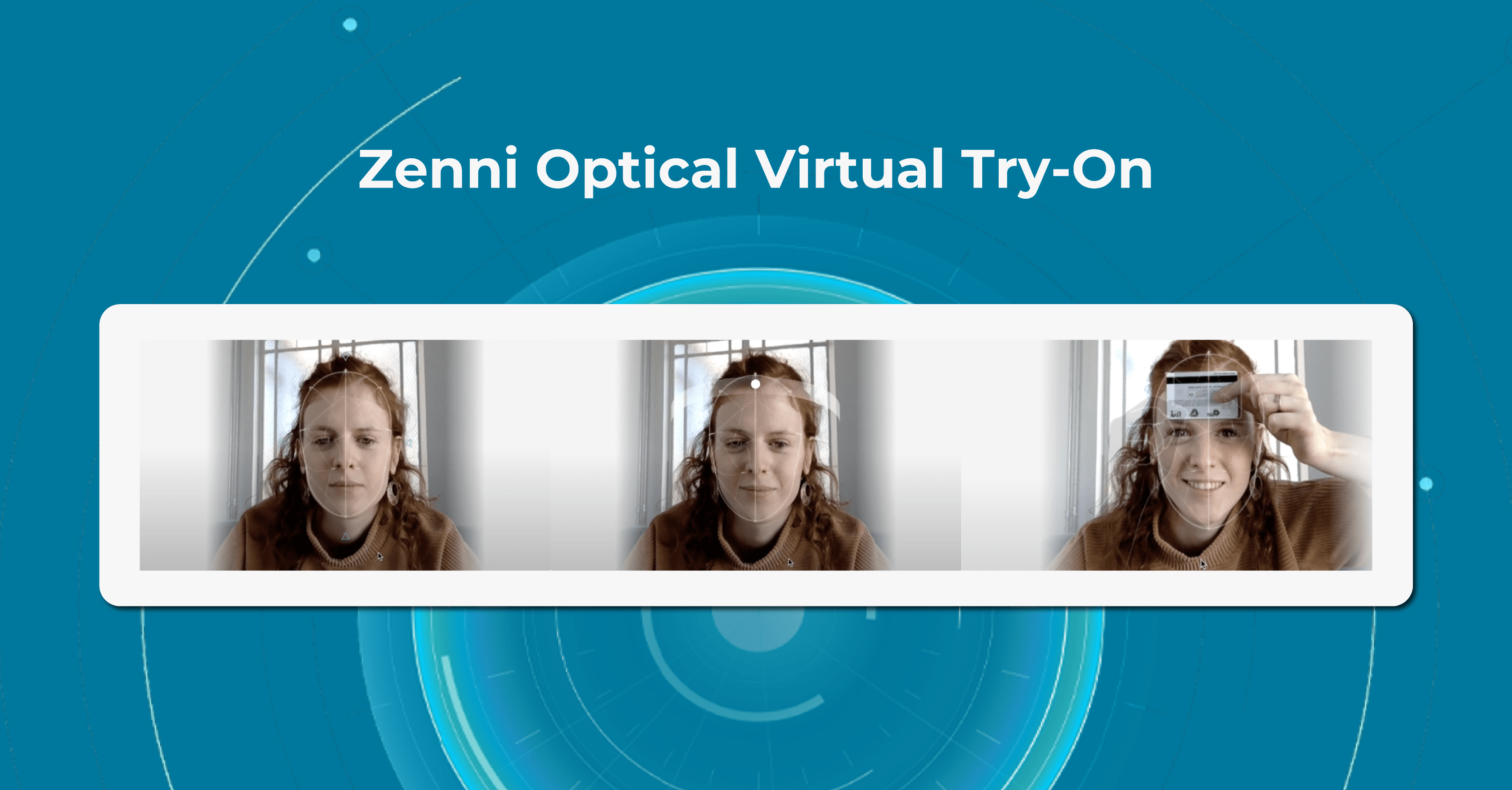
Company’s Virtual Try-On feature uses advanced AR technology to create a 3D model of the user’s face, allowing them to see how different frames will fit and look on them.

To use the Virtual Try-On innovations in eye care, users simply need to upload a photo of themselves or use their computer or smartphone camera to take a live video. This feature then maps the user’s facial features and displays a selection of eyeglasses frames that can be tried on virtually. Users can then select different frames to see how they look from different angles, and can even compare different frames side-by-side.
The Zenni Optical Virtual Try-On is a convenient and easy-to-use tool for anyone in the market for a new pair of glasses. It allows users to see how different frames will look on their faces without the need to visit a physical store or try on multiple pairs of glasses.
VSP Global

VSP Global is a leading eyewear company that was founded in 1955 by a group of optometrists who wanted to provide affordable eye care. Today, VSP Global is a major player in the optometric industry and offers its customers a wide range of services and products.
The company works with a network of over 40,000 eye doctors and optometrists to provide affordable and accessible eye care to its customers. VSP Global also offers other eye care services, such as telehealth consultations, on-site eye exams for businesses and schools, and a mobile eye clinic that serves underserved communities.

AI for OCT
FDA-cleared system for your business
And as every company from this article, VSP Global has a strong focus on technology and innovations in eye care. The company has developed a number of proprietary technologies, including an AI-powered platform called Eyeconic that helps customers find the right eyewear.
Eyeconic uses machine learning algorithms to analyze a customer’s facial features and suggest frames that would fit their face shape and size. VSP Global has also developed a mobile app called myVSP that allows customers to manage their vision benefits, find an eye doctor, and order contact lenses online.
iSight+

Another AI-oriented optometry center is iSight+, located in Hong Kong. iSight+ is an excellent example of how an optometric eye care center didn’t hesitate and chose to provide innovations in eye care and a more in-depth examination of the macula.
Andy Meau. Optometrist, the owner of ISight+ Optometric Eye Care center:
“Altris AI will be a great tool in helping to monitor patients with existing macular diseases. I am also honored to be the first EPC in Hong Kong to provide this service.”
In addition, the eye care center is also equipped with advanced optometric technologies, digital photography systems, and optical coherence tomography (OCT), which helps to provide the highest quality eye examination.
Summing Up
Optometry centers can significantly benefit from incorporating innovations in eye care, such as augmented reality, artificial intelligence, and mobile apps, into their practice. These technologies enhance the patient experience, improve diagnostic accuracy, and streamline clinical workflows.
Moreover, the use of innovative technology can help optometry centers stay competitive in a rapidly evolving healthcare landscape. Patients are increasingly tech-savvy and expect healthcare providers to offer convenient, digital solutions that meet their needs. By embracing innovative technologies, optometry centers can attract new patients and retain existing ones, while also increasing operational efficiency and reducing costs.
Of course, there may be concerns about the cost and complexity of integrating new technologies into an optometry practice. However, the benefits of doing so can far outweigh these potential challenges. With careful planning and implementation, optometry centers can successfully leverage AR, AI, and other innovations in eye care to enhance patient care, improve clinical outcomes, and thrive in a rapidly changing healthcare environment.
-

Future of Optometry: How will Optometry Practice Look in 2040?
 Maria Znamenska
29.03.20239 min read
Maria Znamenska
29.03.20239 min readFuture of Optometry: How will Optometry Practice Look in 2040?
In the next two decades, we can expect to see a paradigm shift in the way optometry is practiced. Advances in new technology, such as AI (artificial intelligence), machine learning, and virtual and augmented reality, are expected to revolutionize how optometrists diagnose, manage, and treat eye-related problems. Optometry’s future is promising for those who are ready to embrace innovations.
For example, smart contact lenses that can monitor blood sugar levels for diabetic patients or detect early signs of glaucoma are already in development, and they could become mainstream within the next 20 years.

In addition to the innovations, changes in demographics will also play a significant role in shaping the future of optometry. The aging population will require more specialized eye care, particularly for conditions such as macular degeneration and cataracts, which are more prevalent in older adults. The rise of chronic diseases such as diabetes will also increase the demand for optometric services, especially in developing countries where access to healthcare is limited.

Register in a free Demo Account to see how AI for OCT works. AMD, DR, early glaucoma examples.
The future of optometry is exciting and holds great promise for patients and practitioners alike. In this article, we will explore some of the potential changes that ODs may face in the coming years based on the survey that we have conducted.
In the next 20 years, the technology in eye care will be represented by AI and is expected to revolutionize the field in several areas. Here are some ways AI is helping in optometry:
- Diagnosis and treatment. AI algorithms can analyze large amounts of patient data and provide accurate and fast diagnoses of eye diseases such as glaucoma, diabetic retinopathy, and age-related macular degeneration. AI could also help in designing personalized treatment plans for individual patients.
- Screening and monitoring. AI can help specialists screen patients for eye diseases more accurately and quickly. For example, a patient could take a picture of their eyes with their smartphone, and an AI algorithm could analyze the image for signs of eye disease. AI could also help monitor the progression of eye diseases over time.

- Enhance patient care. AI-powered tools could help ODs provide more personalized and comprehensive care to their patients. For example, the AI algorithm helps to select the most suitable eyeglasses or contact lenses for a patient based on their unique vision needs and lifestyle factors.
- Research and development. AI could help optometrists develop new treatments for eye diseases. By analyzing large amounts of patient data, AI algorithms could identify new patterns and potential treatments for eye diseases. Enhanced by AI precision, this enables more accurate identification and quantification of biomarkers, leading to better patient stratification, treatment monitoring, and prediction of therapeutic responses.
In addition, the implementation of AI can present various prospects for improving clinic operations, simplifying billing procedures, accelerating the input of EHRs (electronic health records), optimizing claims management, and boosting cash flow. As high-deductible health plans (HDHPs) gain popularity among employers and patients, revenue cycle management can be seamlessly integrated with AI, considering the increasing number of patients defaulting on their medical bill payments.

Although artificial intelligence is about to bring significant changes to the industry, it is important to remember that its effectiveness is limited to tasks that it has been specifically trained to perform. In contrast, AI may not perform well in areas outside its training.
Therefore, it is crucial to focus on enhancing ODs’ proficiency in utilizing AI instead of worrying about the possibility of job replacement. The integration of AI provides specialists with an opportunity to enhance patient outcomes on a global scale.

Register in a free Demo Account to see how AI for OCT works. AMD, DR, early glaucoma examples.
To utilize cutting-edge technologies proficiently, OD specialists must possess critical thinking skills and the ability to manage complex cases in real-time. Additionally, communication skills are essential, including cultural sensitivity, multilingualism, and familiarity with alternative communication platforms such as smartphone-based applications. These skills will be particularly important for optometry specialists in 2040.

Overall, AI has the potential to greatly improve the accuracy and speed of diagnosing and treating eye diseases, leading to better patient outcomes and a more efficient healthcare system.The evolution of OD and MD roles
In 2019, Richard C. Edlow, OD, claimed that nearly 20 million more routine and medical eye exams will be required in 2025 compared to 2015. That is the future of optometry that may look frightening because of the burden. The volume of surgery required for the aging US population will also increase. What is more, the number of cataract surgical procedures will also significantly increase—from 3.6 million in 2015 to 5 million in 2025. Add here the fact that the number of ophthalmologists will increase by only 2.1% in this same period.
Given these facts, in the not-too-distant future, ophthalmologists will need to focus on surgical procedures, while optometrists will provide more medical care.

The field of ophthalmology must be fully prepared to meet the huge and growing demand for surgical procedures and therapeutic intravitreal injections. This brings us to the fact that the field of optometry, in turn, must be ready to manage the ever-increasing demand for medical ophthalmic services.
The roles of OD and MD are changing. With the advent of electronic healthcare, ophthalmologists are already spending more time on the computer than providing proper patient care. The ability to use innovative technology as well as in ophthalmology, digital thought processes, and critical thinking will create new opportunities in eye care as optometrists move further towards ‘data analysis’ and away from ‘data collection.’ OD specialists must ensure that they are properly trained in new technology in optometry and its advances to enhance, not inhibit, the quality of patient care.

It is also worth mentioning that despite the speed of new technology in optometry, the human relationship between patient and doctor remains the most powerful tool. To properly care for patients, ODs will need more than clinical skills, knowledge, or the latest technological advances. Patients need thoughtful, professional, kind, trusting, understanding, and caring optometrists.
As technology for the eye care advances, its education will also change. There may be more need for data analysis, less need for data collection, and an increased need for interpersonal skills (such as empathy, compassion, and bedside manner).
Future of Optometry: AI for OCT technology in optometry
OCT has become an important diagnostic tool for the detection and treatment of various eye diseases, such as glaucoma, macular degeneration, and diabetic retinopathy. Its ability to obtain high-resolution cross-sectional images of the retina and optic nerve will broaden the horizons of technology and help optometrists detect and track changes in ocular structures that may not be visible during normal eye examination.

Here are some ways in which practitioners will benefit from implementing technology in the eye care:
- Improved diagnosis. OCT provides highly detailed images of the eye’s structures, allowing ODs to detect and diagnose eye conditions much earlier than with traditional methods. In fact, OCT is also called an optical retinal biopsy. This method makes it possible to examine 18 zones of the retina and detect minor or rare pathologies. This enables optometrists to provide timely treatment and prevent further damage to the eye.
- Better management of eye diseases is the future of optometry. OCT allows optometrists to monitor the progression of eye diseases such as glaucoma, ARMD, and diabetic retinopathy by taking detailed retinal images. It helps to determine the severity and stage of the disease, compare images after examination with documented results, and track disease progression. Moreover, with OCT examinations, ODs can also monitor the same patient to choose the most accurate diagnosis.
- Enhanced patient care. OCT is a noninvasive and painless procedure that is easy for patients to undergo. It uses safe laser light, avoiding all the side effects or risks. As the procedure is comfortable and effortless for both the ODs and patients, it helps to build stronger relationships by providing a less intimidating experience than other examinations.
- Increased revenue. Optometrists who offer OCT in their practices can generate an additional revenue stream by charging for the procedure and using it to attract new patients.
And, as OCT becomes a standard tool in optometric practice, generating vast amounts of imaging data, AI is perfectly poised to revolutionize how this data is analyzed, interpreted, and utilized to improve patient care.
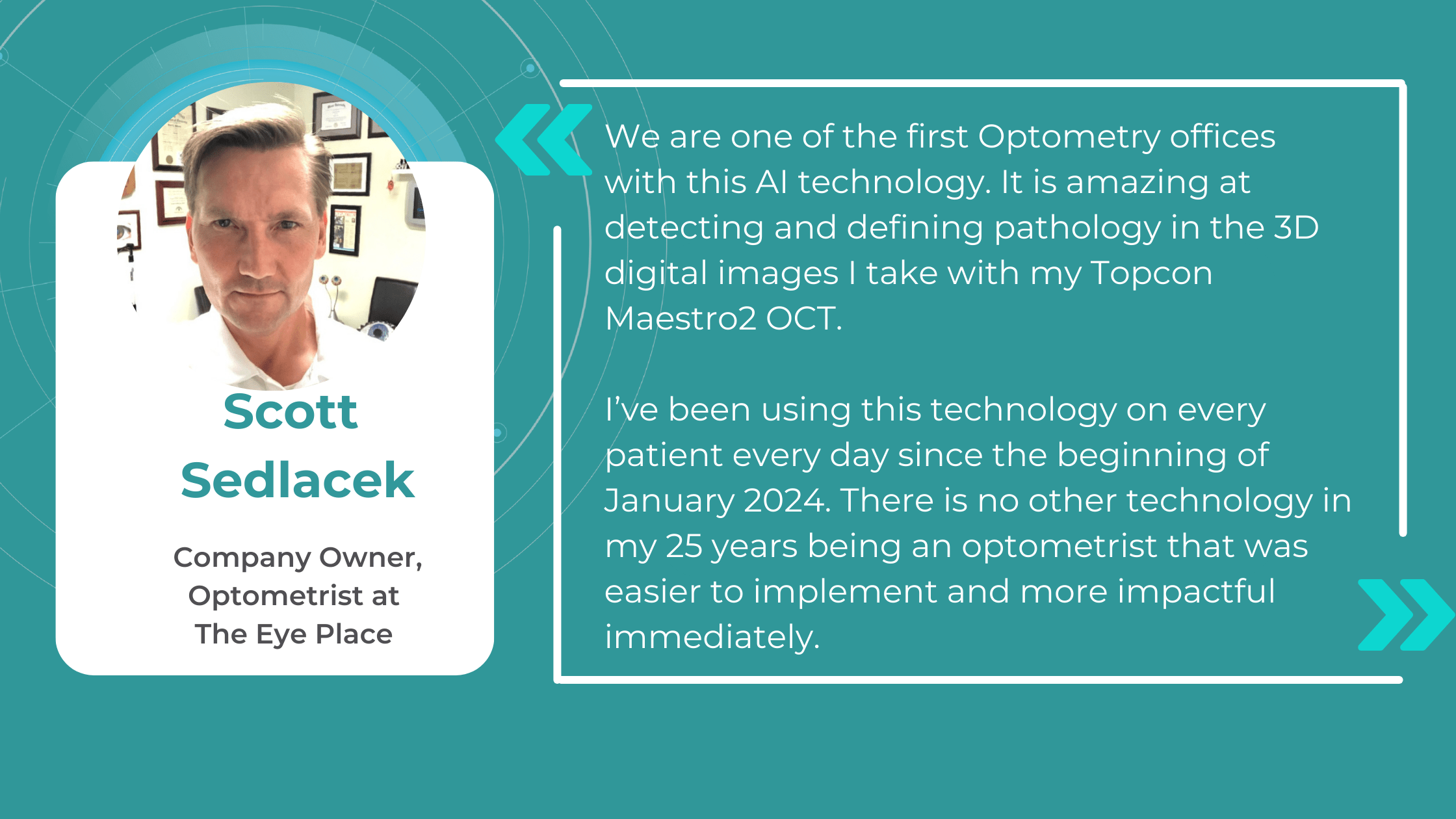
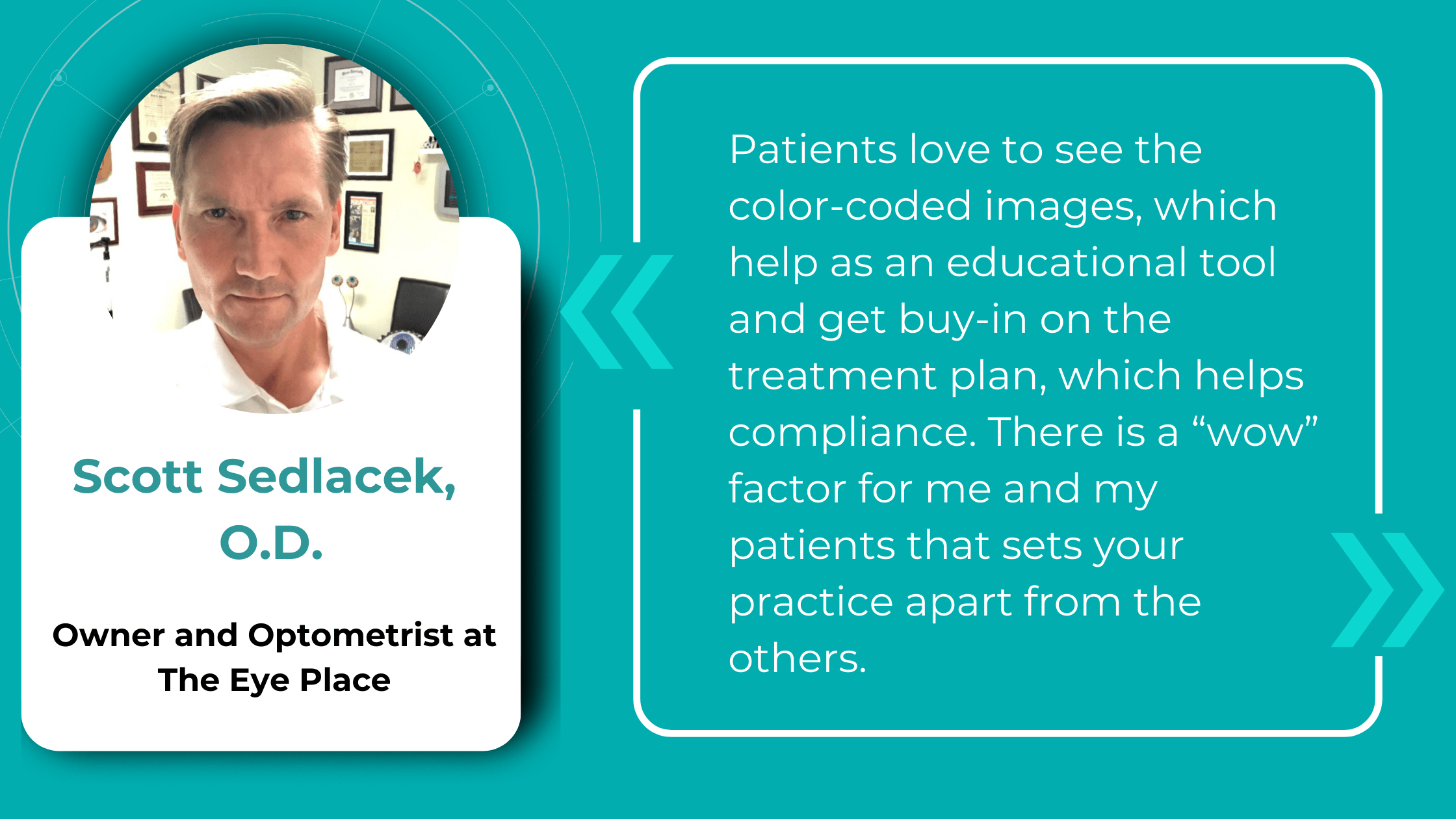
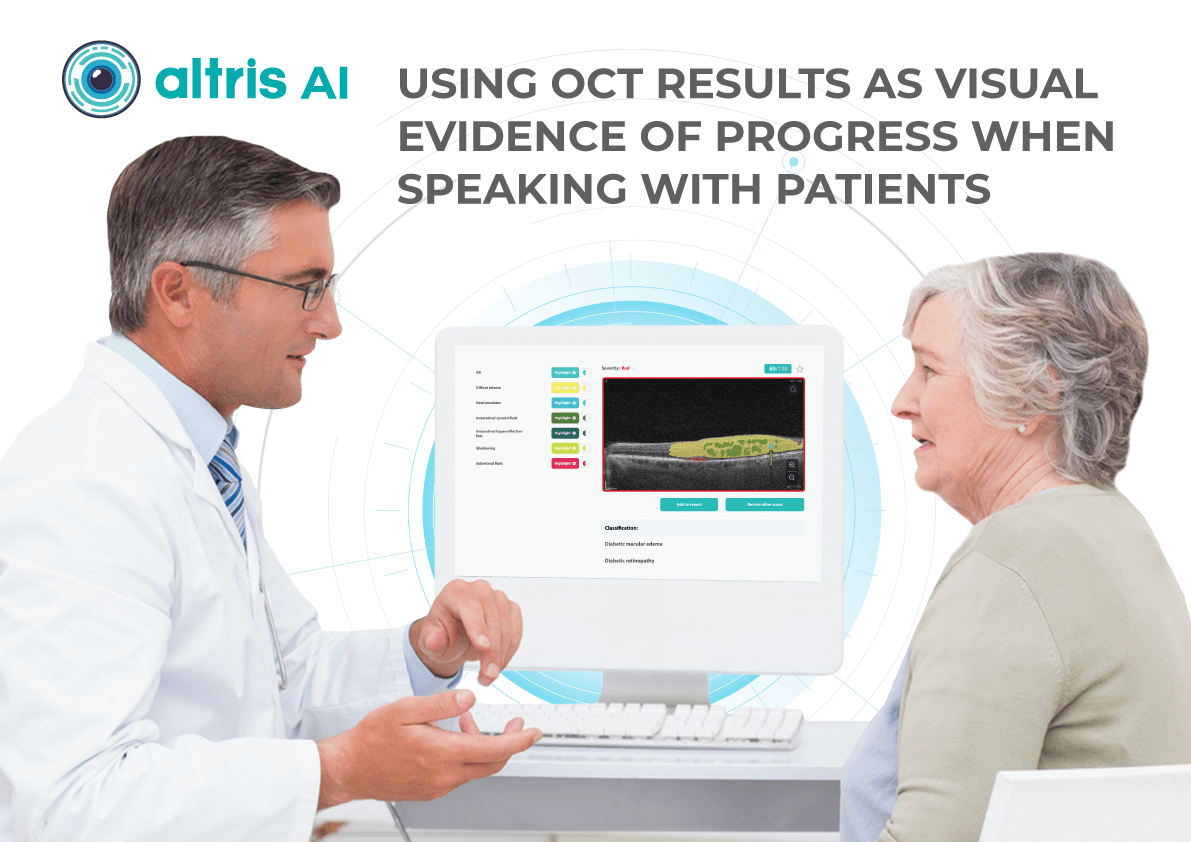
The impact of AI is already being felt in real-world optometry practices. For example, The Eye Place, an optometry center in Ohio, has successfully implemented Altris AI, an AI-powered OCT analysis system. Dr. Scott Sedlacek, the owner of The Eye Place, reports that the system has been instrumental in detecting and defining pathologies that he might have missed, leading to earlier intervention and improved patient outcomes. Patients also appreciate the color-coded images generated by the AI, which serve as an educational tool and help them understand their treatment plans better.

AI technology in optometry is improving diagnostic accuracy and enhancing practices’ overall efficiency. By automating tasks such as image analysis and data entry, AI frees up optometrists’ time, allowing them to focus more on patient interaction and complex decision-making. This streamlined workflow not only benefits practitioners but also improves the patient experience, making integration of AI into optometric practice not just a possibility but a new standard.
The future of Optometry: Focusing on myopia management
According to a survey conducted by the American Optometrists Association, nearly 70% of optometrists reported an increase in patient requests for myopia treatment in the last two years. Myopia is a rapidly growing problem worldwide. Only in the USA, it is predicted that by 2050 the number of patients will increase to 49.8%. As unfortunate as it may be, such a global epidemic of myopia will undoubtedly create an opportunity to expand the practice of specialized treatment.

In the future, optometrists may manage myopia using a combination of approaches, and one of the most discussed is orthokeratology (ortho-K). This non-surgical approach that involves wearing specially designed contact lenses has been used to reduce the degree of myopia since the 1960s. Although this method is not new in optometry practice, many companies are still working hard to create new approaches and upgrade them. For example, two years ago, Johnson & Johnson Vision announced FDA approval of its Acuvue Abiliti Overnight Therapeutic Lenses for the management of myopia. That same year, CooperVision announced that its Procornea DreamLite night lenses for ortho-k had received the CE Mark from European regulators for slowing the progression of myopia in children and young adults.
Overall, the future of myopia management with new technology in optometry will likely involve a personalized, multi-faceted approach that combines various strategies to reduce the progression of myopia and improve vision.
Game-changing contact lenses
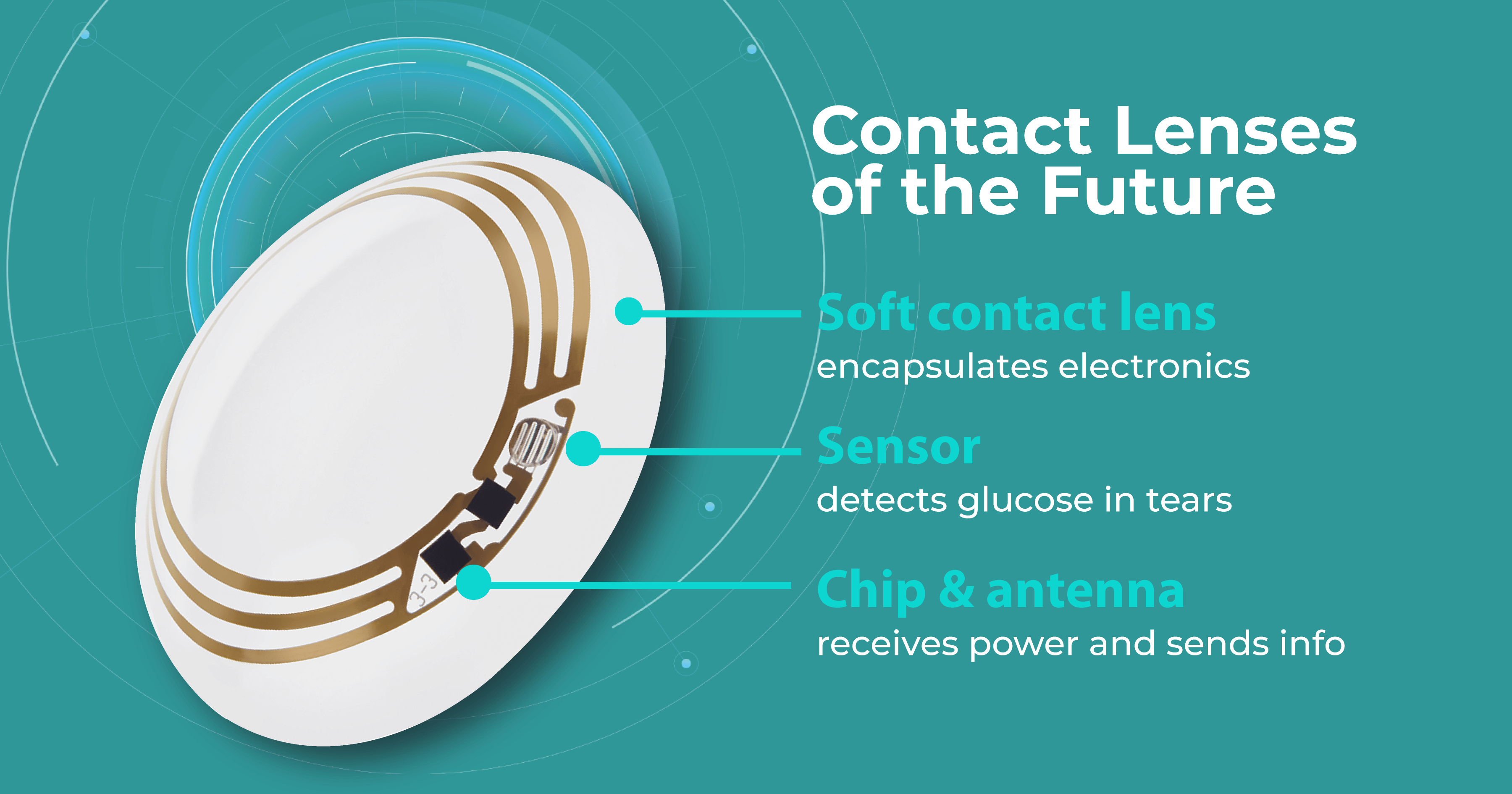
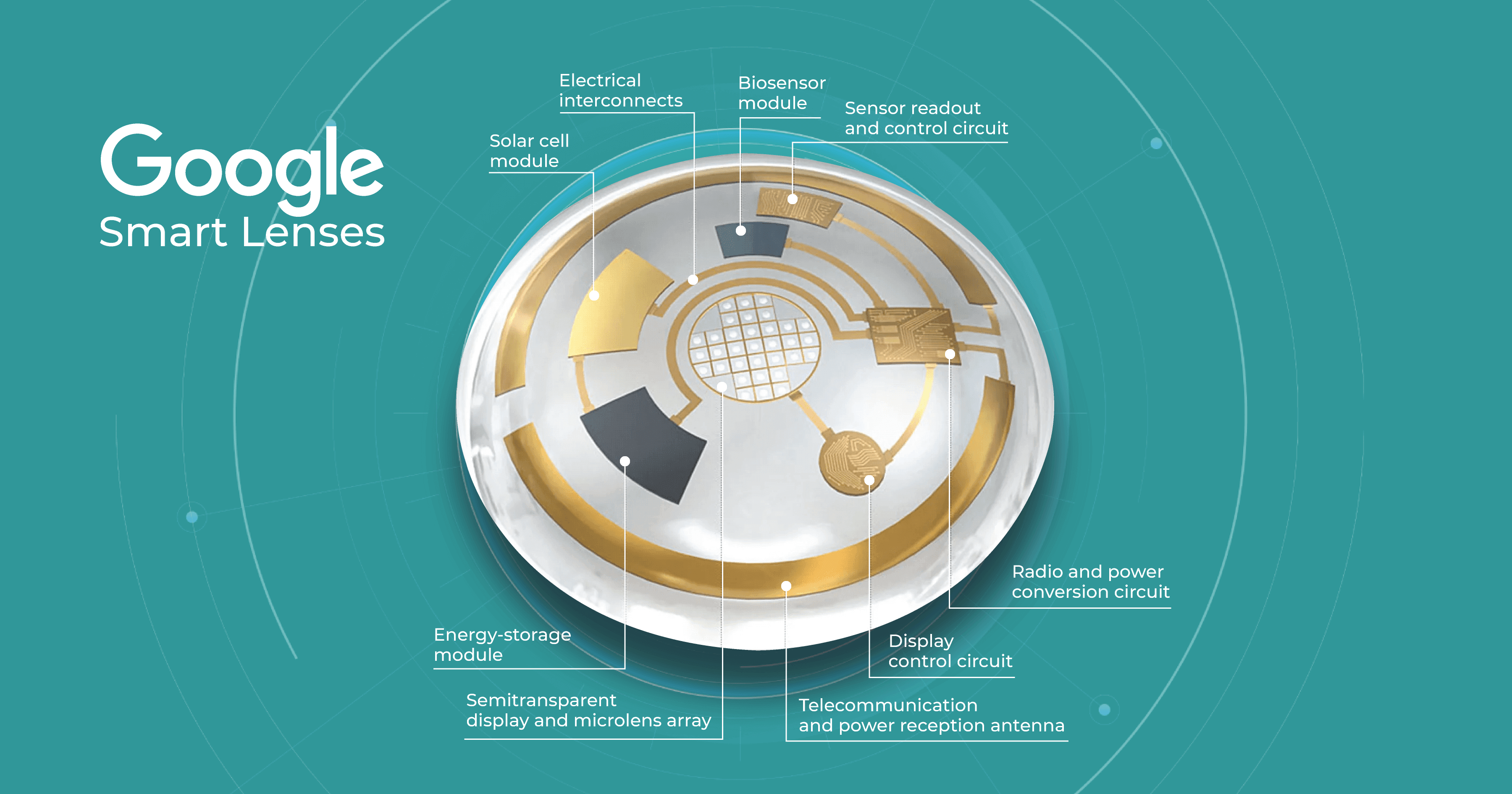
Research published in Advanced Materials Technologies claimed that contact lens sensors can be used to monitor many common diseases in the near future. The fact is that biomarkers in the lacrimal fluid make it possible to create diagnostic contact lenses. Such lenses would analyze these biomarkers and detect and treat systemic and ocular diseases such as diabetes, cancer, and dry eye syndrome.
It is predicted that in the near future, lenses will be able to monitor intraocular pressure, detect glaucoma, and even create images of retinal vessels for early detection of hypertension, stroke, and diabetes. For patients with diabetes, these lenses would be incredibly useful because they measure blood glucose levels. Some companies, like Google, have already dedicated years to creating such lenses. Nowadays, scientists are even working on lenses that change color to alert about changes in glucose levels.

However, according to Advanced Intelligent Systems, one limitation of these lenses to date is that they can typically only detect one biomarker in the eye, such as glucose or lactic acid. Lenses capable of detecting multiple chemical components are predicted to be developed in the future.
Summing up
Predicting the exact way new technology will affect optometry practice in 20 years is challenging, as technological advancements and societal changes can rapidly alter the way healthcare is delivered. However, the widespread adoption of AI in optometry is likely to occur well before 2040, making it crucial for practices to consider integrating this transformative technology now to remain competitive and provide cutting-edge care. Nevertheless, even though AI and technology will gain popularity among eye care specialists, AI and machine learning will still be only assistants. At the same time, ODs will be responsible for diagnosis, treatment, and care.

Check how artificial intelligence assists in OCT interpretation
This brings to the forefront the important principles of patient education, empathy, and personal contact with patients (virtue ethics). Innovations in optometry technology should allow ODs to have more personal contact and more time to improve outcomes for patients-not to improve productivity.
In addition, optometric education will need to address these interpersonal skills so future generations of ODs are able to adequately educate patients on findings and ensure the quality of care.
There will always be a business of health care, but the challenge for the optometric profession is for ODs to prioritize the well-being of all patients.
-

Optometry Practice Management Tips: 10 Real Cases for Revenue Increase
 Maria Martynova
14.02.20236 min read
Maria Martynova
14.02.20236 min readOptometry Practice Management: Tips and Real Cases
You’re a skilled optometrist, passionate about patient care. But are you prepared for the challenges of running your own practice? Successfully navigating the business side of optometry practice management demands more than just clinical expertise but also a deep understanding of business management principles. This transition involves constant decision-making, from choosing the right location and equipment to hiring and managing staff.
We’ve gathered information on ten optometry centers that managed to survive the competition and increase their revenue, as well as optometry practice management tips. The articles will guide you through major challenges that many optometry businesses face, such as the retention of specialists, competition with large chains and retailers, and marketing and sales, identifying growth opportunities.

AI for OCT analysis
Make your eye care business technological
How to improve optometry practice: start with the retention of employees
This problem is vital considering the huge lack of optometry specialists worldwide. According to WHO, 14 million optometrists are needed globally when there are only 331K available. There are several strategies that optometry businesses can use to retain optometrists.
- Delegating more examinations to technicians
Eric Rettig, OD, a partner with Mountain View Eye, a Vision Source practice in Pennsylvania, shares their optometry practice management optimization: assigning two technicians to each of its four doctors to delegate the examination process to the technicians. Tasks such as pupil testing, versions (EOMs), and dilation were incorporated into the pretesting protocols. Technicians were also authorized to perform additional testing based on patients’ past diagnoses or complaints. This approach ensured that the doctor had all pertinent data readily available upon entering the exam room, minimizing patient wait times and maximizing efficiency.
Owners have implemented the change to increase the number of patients seen per hour, but it has also given additional benefit: now doctors can spend more quality time with each patient.

With an average revenue of $400 per patient and 6-7 patient care hours per day in a five-day week, this equated to $2,600 additional revenue per full-time doctor.
- Using Artificial Intelligence for retina scan analysis.
Many optometrists find OCT scan analysis challenging and are not confident about their interpretation skills. Using Artificial Intelligence for automated OCT scan analysis can make the work of optometrists more efficient, increasing the number of patients who undergo OCT examination and subsequently increasing revenue.
One such case is the practice of Dr. William C. Fruchtman’s Optometry, owned and operated by Dr. William C. Fruchtman, O.D., in New Jersey.
His practice offers comprehensive eye care services, including eye examinations, contact lenses, and glasses prescriptions. Dr. Fruchtman sought a tool to enhance both his optometry practice management and decision-making process in complex cases. His research led him to select Altris AI, an artificial intelligence platform for OCT scan analysis.

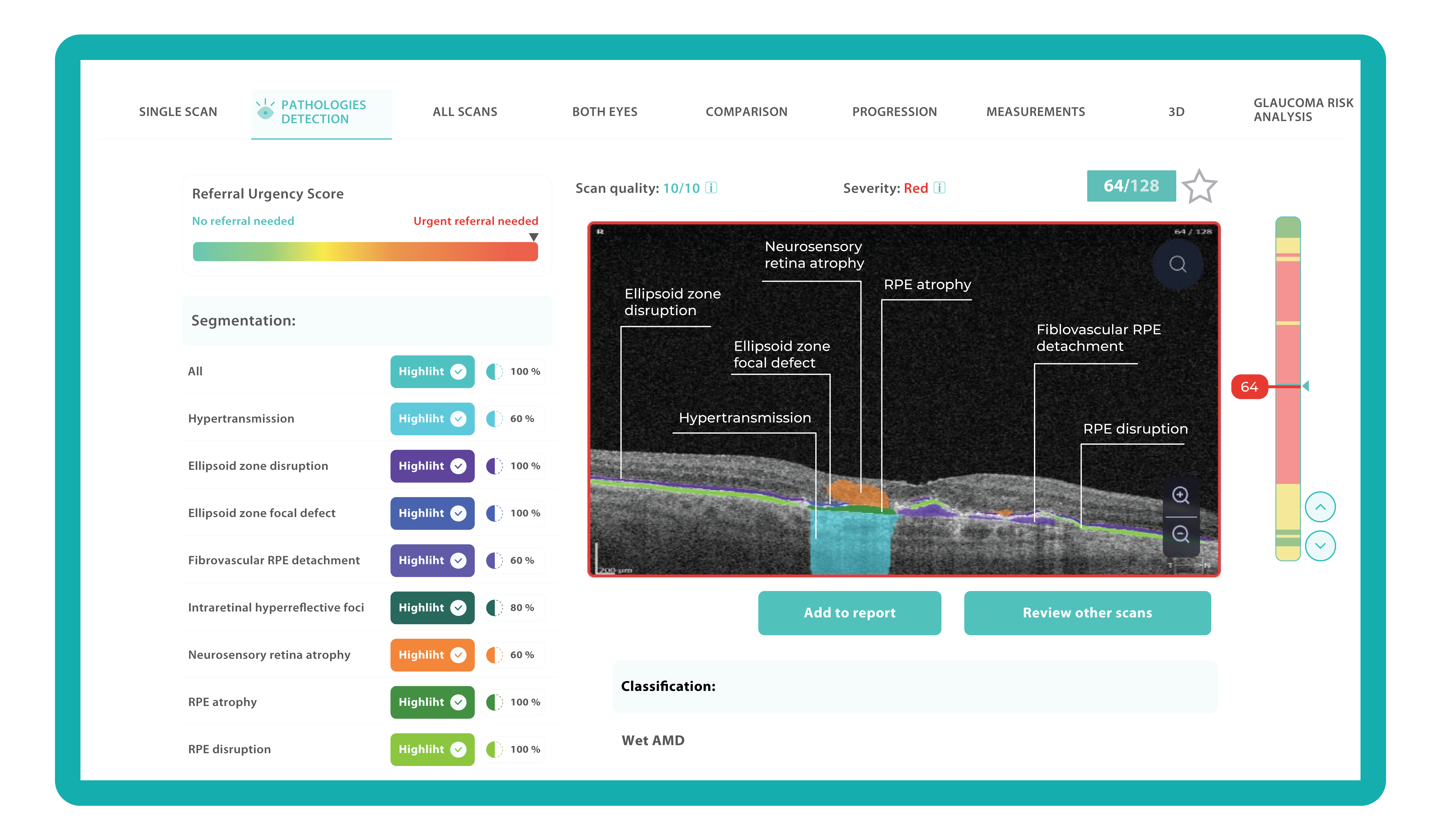
Implementing Altris AI has significantly increased Dr. Fruchtman’s confidence and precision in diagnosing and managing eye conditions. The platform has also provided his practice with a competitive advantage. Altris AI features a referral urgency score ranging from green (no need to refer) to red (urgent referral needed). This scoring system helps optometrists avoid both over-referral and under-referral of patients.
Thanks to the color-coded and labeled OCTs, optometry facilitates patient education and enables practitioners and patients to monitor the progression or treatment results more effectively.

Optometrist Marketing: digital communication trends
- Concentrating on eyewear sales.
Jennifer Stewart, O.D., Optometrist and Founder at Look New Canaan, Connecticut, claims that 2 simple optometry practice management techniques can add $75,000 to the annual revenue of any optometry center. Even more intriguing is that it’s done without seeing additional patients.
One of these techniques is decreasing the sales of patients’ own frames (POF) glasses. Jennifer Stewart discusses the benefits available to the patient through their managed care plan, emphasizing that if lenses are cut for their own frame and the frame breaks, they will have already used their lens benefit.

The optometrist explains how the patient’s current pair can serve as a backup and then escorts them to the optical area to meet with the optician. Before leaving the patient with the optician, the optometrist speaks privately with the optician, informing them of the patient’s desire to use their own frame and the discussion about the frame’s condition. The opticians have been trained to reiterate this message to the patient.
The second one is communicating the need for all types of lenses (for computers, reading, and sunglasses), which can be a very effective revenue-generation optometry practice management tip many owners neglect. The optometrist states that these few extra minutes to talk about options available to patients can result in multiple payoffs in optical. This is one of the optometry practice management tips that works for any center.
- Providing exquisite luxury experience.
Fabio Pineda, the owner of Eye Boutique in Houston, Texas, previously held a volume-based, medical-style practice with an average per-patient purchase of one frame per year, 5 percent sunglass sales, and an average per-patient revenue of $300-$350. In 2021, the optometrist changed his approach, opening a fashionable boutique-style practice. He shifted to a low-volume VIP clientele and a red-carpet approach with gourmet beverages, pastries, and a dedicated sunglass section with a wide selection.
This shift in eye care practice management has brought Dr. Pineda unique customers who specifically seek designer glasses and buy 5-9 pairs at a time, spending upwards of $4,000-$7,000 on purchases.

- Using social media and digital marketing tools extensively.
Your clients spend time on Instagram, Facebook, and Google, so these are the most effective digital marketing channels for communication with potential customers.
For instance, as an optometry marketing strategy to engage current and potential patients on social media, Dr. Arian Fartash, optometrist, CEO at GlamBaby, California, and blogger, considers three types of posts:
- interactive posts that pose questions about product preferences, like showcasing two frames and asking followers which they prefer, encourage audience participation;
- educational posts featuring interesting eye facts or eye-catching images related to eye health that offer informative content;
- patient-focused posts showcasing satisfied patients wearing new eyewear to humanize the practice and demonstrate the positive impact of its services.

- Providing a small warranty on all products.
An optometrist and the owner of Brilliant Eyes Vision Center in Georgia, Janelle Davison, O.D., has implemented an extended warranty program for eyewear purchases to enhance patient confidence and increase revenue. For a $29.99 enrollment fee, patients receive significant discounts on replacement frames and lenses, paying only $50 for each, regardless of the original cost. This warranty, built into most eyewear packages, has proven popular with patients and generated an additional $14,000 in 2021. Dr. Davison has used the popular concept of buying technological equipment with a warranty, like smartphones or computers, that is familiar to customers.

- Educating patients
According to Wolters Kluwer Health, patients crave educational materials from their providers, yet only two-thirds get them. This leaves patients searching for information, potentially exposing them to unreliable sources.
Knowing that providing clear, accessible patient education is crucial for understanding and treatment adherence, The Eye Place, optometry from Ohio, is utilizing the full power of AI for OCT analysis tool, Altris AI. Their winning optometry practice management strategy combines decision-making help from the platform and a way to enhance patient education.

Visual representations of patients’ conditions, facilitated by this technology, empower patients with a clearer understanding, leading to increased treatment compliance.
Eye Care Practice Management: competition with larger chains
Private offices find it hard to compete with chains like Specsavers in terms of prices or the speed of service. Chains often have better locations and can spend much more money on marketing. So, how to improve optometry practice to win this competition among corporations? There are several things that big companies don’t have:
- Offering personalized service and building a relationship with patients. Building a local presence is the key. Your optometrist center can be known and valued if you really care about the community, know each of your clients personally, and understand their pains and needs. More than that, 97% of marketers witnessed a rise in business outcomes as a result of personalization, according to Salesforce.
- Providing unique, high-quality products unavailable at chain stores is also a worthy opportunity for a small but flexible business. For instance, some optometry centers build their presence relying on rare glasses brands with sophisticated designs. The global therapeutic contact lenses market is expected to grow at a CAGR of 4.90% from 2021-2027, and designer brands will play a crucial role in this growth.
- Providing exceptional customer service and after-care. Communication with customers is the core of relationships in any sphere, and healthcare is no exception.
Today it’s easier to communicate with customers using social media, messengers, and telemedicine. This is the one of optometry management tips that not only allows optometry centers to take care of their clients not only during visits but afterward as well are much more profitable.

AI for OCT analysis
Make your eye care business technological
- Storing all the patients’ data effectively and securely is the key to fast and reliable services inside the optometry centers. There are various EHR systems for optometry centers, and finding the best optometry practice software is hard. However, it is always wise to rely on testimonials. Here, you can find another portion of optometry practice management tips that focus solely on the best optometric practice management software with Acuitas activEHR 2.0, MedFlow EHR, Liquid EHR, EyePegasusEHR, Eye Cloud Pro, OD Link, ManagementPlus, Medesk named the best optometric practice management software according to our research and reviews.
PATIENTS’ NO SHOWS
A patient no-show is a painful problem for the majority of optometry centers. Patients ignore yearly checkups and forget about follow-up visits whenever they feel better.
Virtual check-ins increased profitability and reduced the cost of goods sold (COGS) for the partner at Wichita Optometry. Dr. Chad Fleming adopted this optometry practice management approach through the efficient check-in process he observed at Walmart. His practice faced the challenge of managing a high volume of phone calls and text messages, requiring either additional staff hiring without an immediate increase in revenue or a strategic reallocation of existing personnel.

Using software to remind about future visits can be the solution. For instance, Weave software helped Serenity i Care optometry to reduce the number of no-shows up to 30% from 75%. This software automatically informs clients about future visits via e-mails and texts.

There is no need for a team to have endless calls that are not responded to. Demandforce, Solutionreach, and Simplifeye are other solutions that might work, and they can be great software for reminding patients about visits. This is the most well-recommended optometry practice management software to deal with forgetfulness.
By using these optometry management practice tips and continuously seeking ways to improve patient engagement, streamline operations, and increase efficiency, optometry can increase its revenue and sustain long-term success.
-

Optometry Practice Management Software: Top 8 Applications
 Mark Braddon
13.02.20239 min read
Mark Braddon
13.02.20239 min readOptometry practice management software is designed for eye care specialists to manage their practices more efficiently and effectively. The software can automate a wide range of administrative tasks, making it easier for practitioners to focus on patient care.
Unlike other medical practices, optometry involves the management of a much larger number of optical instruments, processes and aids. Therefore, software for optometrists is more complex and multifunctional. It usually includes features such as appointment scheduling, patient registration, billing and insurance claims processing, patient data management, and secure messaging and email communication. The software can also integrate with other technologies, such as electronic health records (EHRs), OCT image management systems and diagnostic equipment.

AI for OCT scan analysis
Register in a free Demo Account to see how it works: OCT scans with AMD, DR, early glaucoma are already inside.
By streamlining administrative tasks and providing practitioners with patient data, optometry practice management software can help eye care clinics improve their operations, increase efficiency, and provide better patient care. The software can be customized to fit the specific needs of individual practices and is often offered on a subscription basis, making it an affordable and accessible solution for eye care clinics of all sizes.
In this article, we will highlight the main benefits of practice management optometry soft, and provide you with a list of the Top 8 software to look at.
What are the benefits of practice management optometry software?
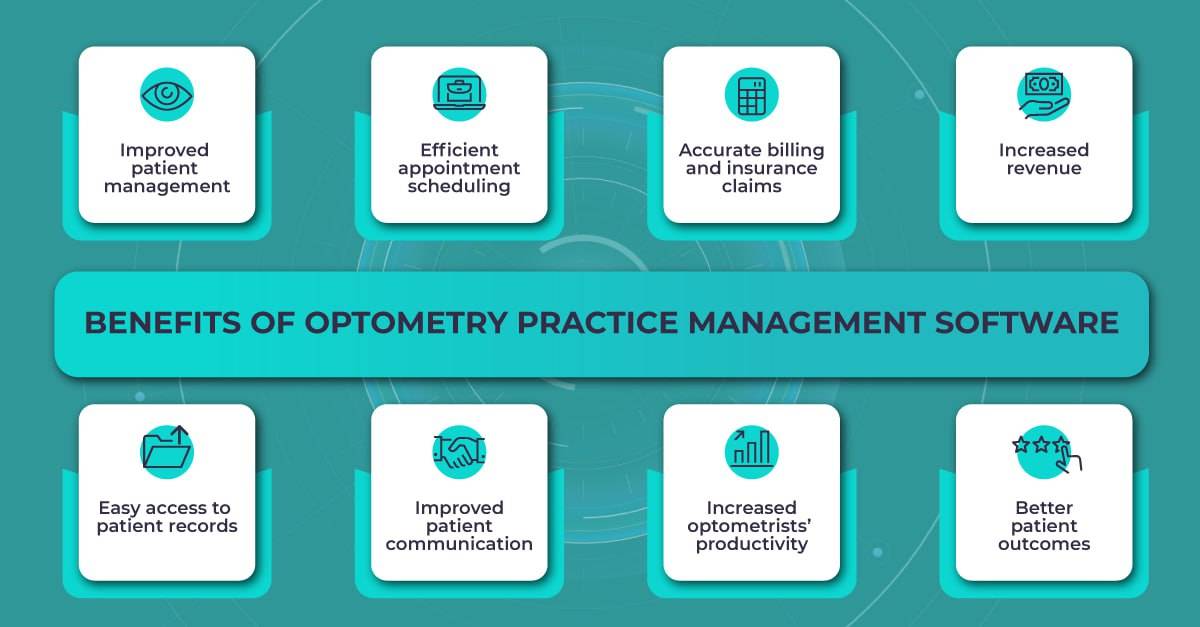
Optometry practice management software can help doctors in multiple ways besides increasing their revenue, efficiency, and productivity. Some of the key benefits of optometry practice management software include the following items.

- Improved patient management. The software can store and organize patient data, including medical history, examination results, fundus or OCT images, and treatment plans. This information can be easily accessed by practitioners and used to inform patient care.
- Efficient appointment scheduling. The software can automate appointment scheduling, which can help to reduce the risk of double-booking and minimize wait times for patients.
- Accurate billing and insurance claims. The software can help to ensure that billing and insurance claims are processed accurately and efficiently, reducing the risk of errors and delays.
- Increased revenue. By streamlining billing and insurance claims processes, optometry practice management software can help eye care clinics to reduce errors and increase revenue.
- Easy access to patient records. The software can store and organize patient records, including OCT images, making it easy for doctors to access the information they need to provide the best care possible.
- Improved patient communication. Some optometry practice management software includes features that allow for secure messaging and email communication between patients and practitioners, making it easier to communicate outside of office visits.
- Increased productivity. By automating repetitive tasks, such as appointment scheduling and billing, optometry practice management software can free up time for eye care practitioners to focus on providing an individual approach to each patient.
- Better patient outcomes. With access to patient data and treatment history, eye care practitioners can provide more informed and effective care. This can lead to better patient outcomes and increased patient satisfaction.
Overall, optometry practice management software can help eye care clinics to provide better patient care, increase efficiency and productivity, and improve their bottom line. Now let’s take a look at best optometry software.
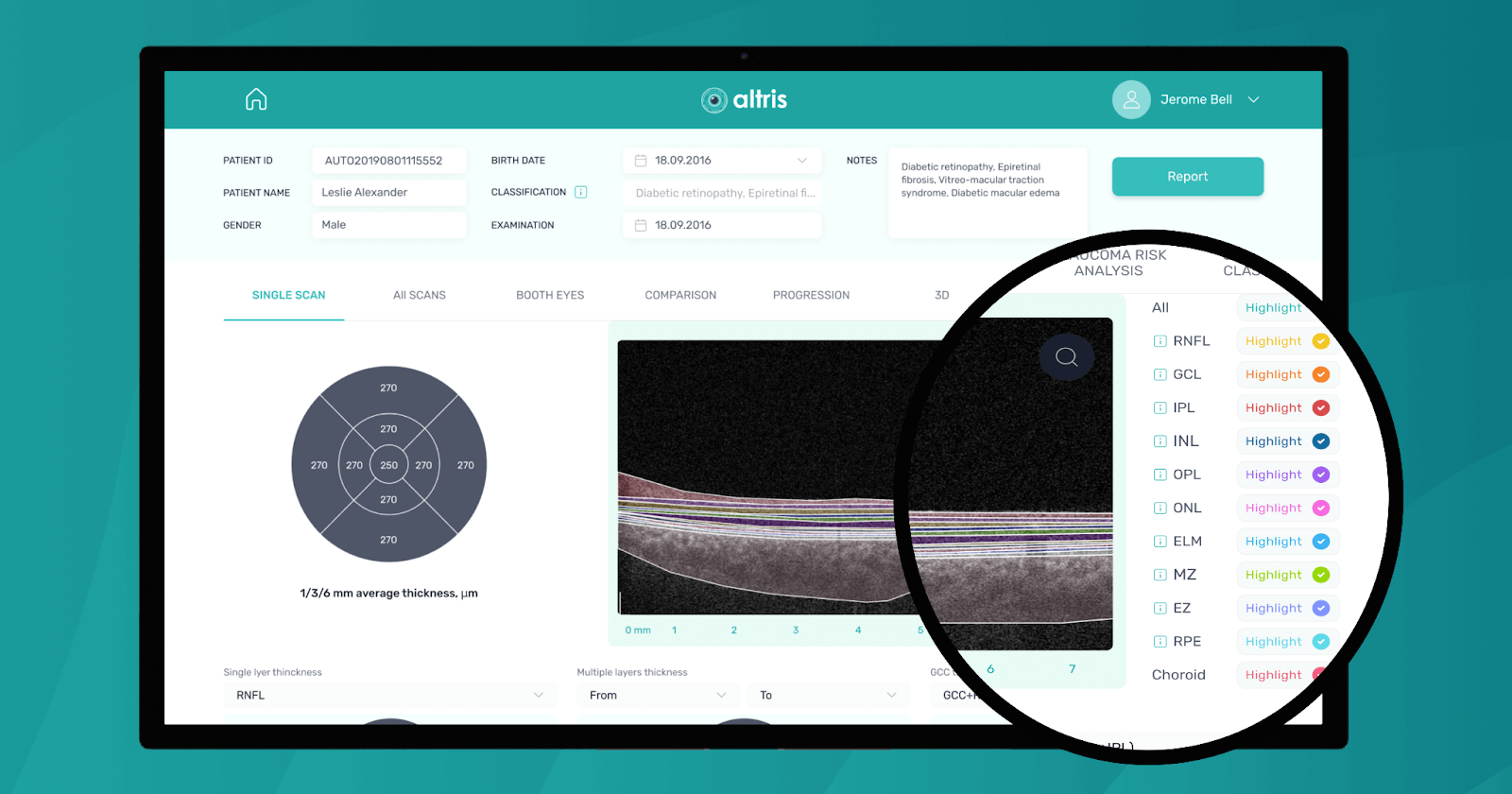
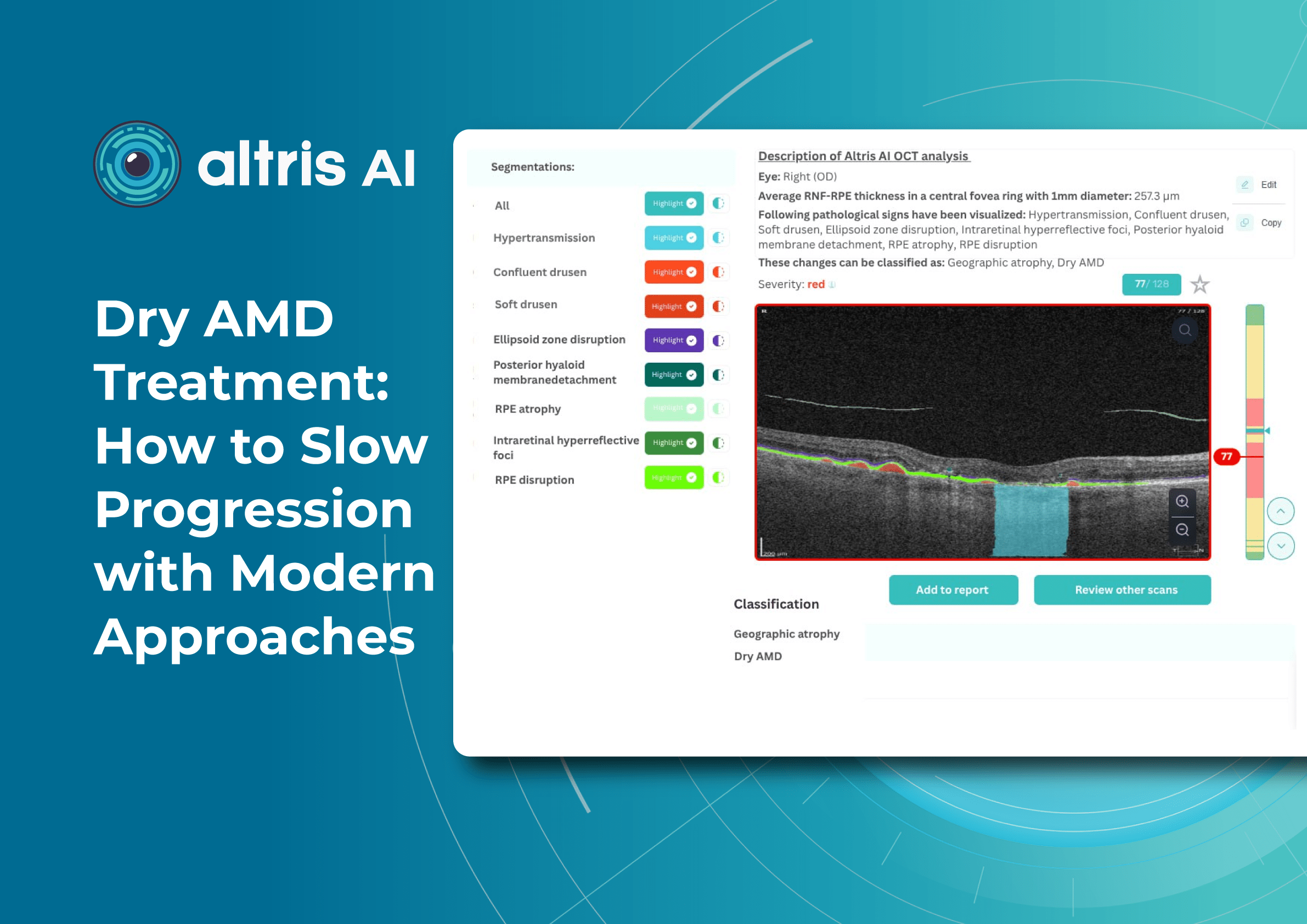
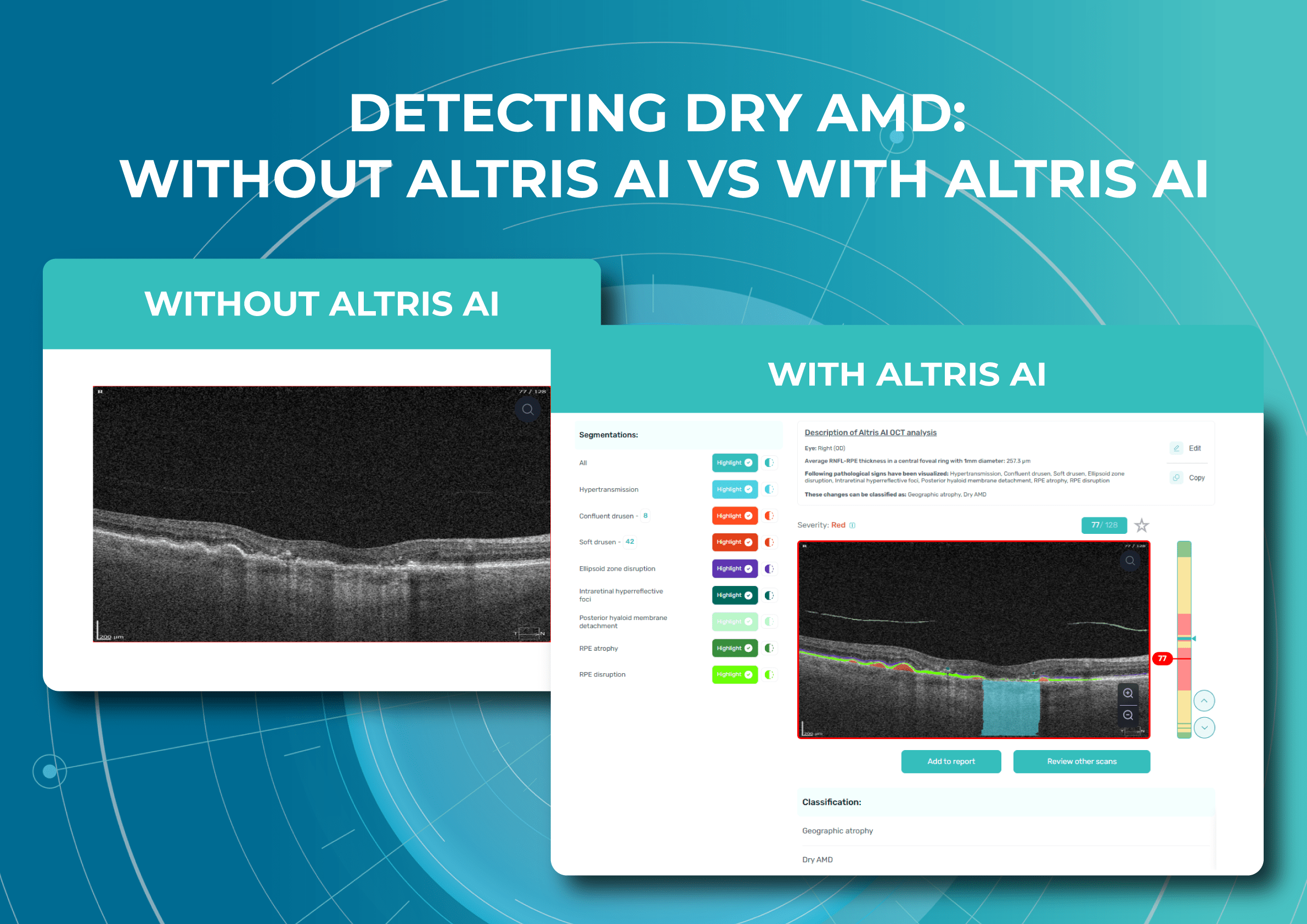
Altris AI

Altris AI is an image management system based on artificial intelligence (AI) tools that assists eye care specialists in OCT scan analysis and interpretation. The solution was designed in cooperation with retina experts to help practitioners detect the pathology from the OCT scan. Altris AI also can be easily integrated with EHR systems or used standalone as a web application.
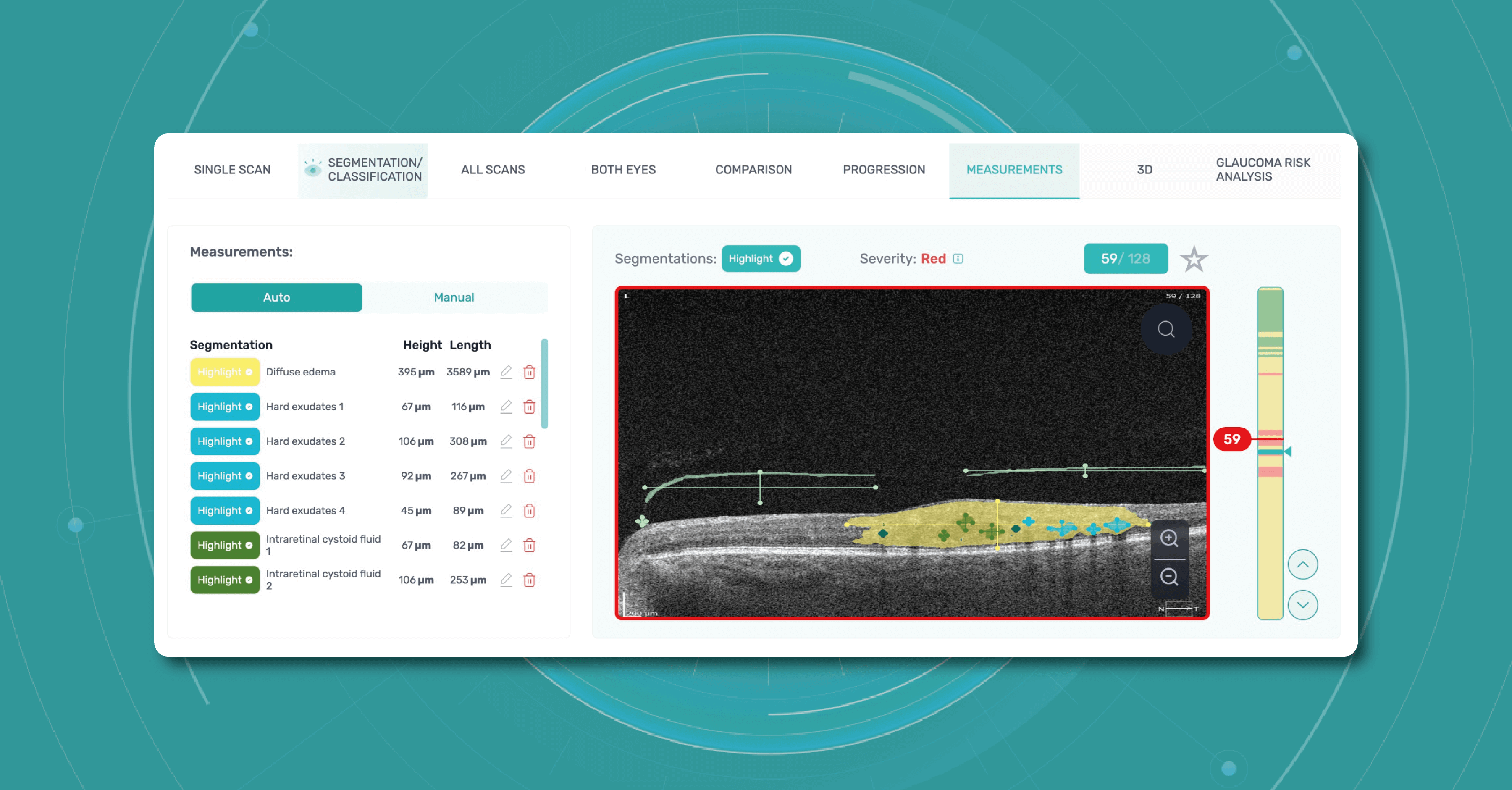
To create an Altris AI system, our specialists colored thousands of OCT scans and named more than 100 retinal pathologies and pathological signs to train an AI algorithm. May sound complicated, but the workflow of the image management system is pretty simple.
- First, a user uploads an OCT b-scans to the platform, and the AI model evaluates the scans.
- After that, the model differentiates between normal scans and scans with moderate and severe pathology.
- With the help of the second step, eye care specialists are able to focus only on serious (red) scans, saving their precious time.
- After that, a user can highlight pathological signs with different colors, sort scans by severity level, and zoom.
It is important to mention that the patient’s diagnosis is always on the eye care practitioner’s side. Altris AI is a tool that provides assistance in support in decision-making and allows its users to see a broader perspective of a patient’s eye health.
Watch a short overview of how Altris AI assists eye care specialists with OCT diagnosis.
In addition, with Altris AI, users can work with all modern OCT equipment and popular data storage formats, such as DICOM of various lengths, png, and jpg. The patient data at all stages is tokenized and protected from disclosure. Eye care specialists can also actively use the Smart Reports feature, which allows users to select a single element (scan, layers, both eyes, etc.) that they want to see in their OCT report.
Acuitas activEHR 2.0

In case you are working at or owning a midsize or large optometry practice, this hybrid electronic health record solution will be quite useful. Acuitas activEHR 2.0 can be hosted in the cloud as well as deployed on-premise, depending on your preferences. This software offers its users a wide range of tools, including electronic medical records, billing software, scheduling, PACs, accounting software and billing services.
What is more, Acuitas activEHR 2.0 can provide optometry clinics with various marketing and upselling features, and you can also customize BI reporting and track benefits. Healthcare providers can reach out to patients via either SMS or email, which makes it much easier to schedule an appointment.
In addition, the optometry practice management software supports such features as IDA (Immediate Data Access), which allows practitioners to automatically update the frames. Acuitas activEHR 2.0 also offers a variety of application integrations.
MedFlow EMR

Next on our list — Medflow EMR software, which was designed to serve as either a standalone EMR (electronic medical record) or as a combination of EMR + practice management (PM) system. Like other optometry practice management software from our list, Medflow EMR was created specifically for eye care, but it can be used by eye care specialists providing both ophthalmology and optometry.
Medflow has a bunch of features, but the main one is the software has built-in templates designed for comfortable and time-saving work, including retina scans and surgery, cataracts, glaucoma, digital drawings, eye measurements, LASIK procedures, and more. In addition, it also has a base package, where ASC and optical modules are included.
Overall, this practice management software will suit a clinic of any size, be it solo practice or a large hospital. The Medflow interface can be easily integrated with other practice management systems or image interpretation applications. Also, the software can be used as a hosted solution or installed on-premise.
Liquid EHR

Liquid EHR software will be a perfect solution rather for small and midsize optometry practices than large hospitals. The broad range of its features includes medical records management, medical billing, scheduling and a lot more. The optometry practice management software provides eye care specialists with the ability to generate a mailing list, track systems workflow, manage documents, do compliance checks, integrate e-prescribing, and configurable exam records.
What is more, Liquid EHR has a number of specific optometry tools, such as historical IOP charts, drawing tools, built-in eye charts, frames data integration and image management. Optometrists can incorporate lab test results, view clinical summaries and send patient reminders.
In addition, the software also allows practitioners to have instant access to electronic insurance filing tools, patient recalls, drug interactions and allergy interaction checks, problem lists, active medication lists, medication recommendations, educational resources, smoking status, vital signs and more.
EyePegasusEHR

The EyePegasus optometry practice management software offers a solid number of tools and features for optometry practices. You can schedule appointments online, turn on the automatic appointment reminders, work with a patient portal, scan documents, use an optical calculator and an iOS app with patient check-in features.
Using EyePegasus, eye care specialists can customize different tabs by choosing a proper layout, and create templates for treatment documentation. Moreover, optometrists are able to scan medical images and upload them directly into a patient’s chart. The is also a possibility to create referral letters using auto-populated EHR data. Other EyePegasus tools include building and dispensing optical orders and online appointment management.
In addition, the optometry practice management software allows managing inventory of different items, such as lenses. EyePegasus also can be integrated with a variety of applications.
Eye Cloud Pro

Another optometry practice management software created for optical professionals is Eye Cloud Pro. The list of its data managing tools is really impressive and includes e-prescribing, inventory management, integrated credit card processing, electronic claims submission, device integrations, two-way texting (SMS), and ECP Billing.
The system also provides improved patient communication via secure messaging and email services. Clinic managers can configure various appointment types and lets clients request bookings via mobile or desktop devices. The software can be customized mailing lists, referral reports, account information, and sales reports to help with business strategy.
In addition, one more benefit of Eye Cloud Pro software is that it has an integrated payment processing system with automated invoice and receipt generation. It will make a clinic’s data safe and retained.
OD Link

Taking about comprehensive optometry practice management software, OD Link is one of the most suitable variants for any clinic. It has both PM and EMR/EHR tools, helping to manage patient records, exams, appointments, inventory, billing/insurance information, and much more.
OD Link software allows optometry practitioners to communicate with patients via SMS or email, work with electronic insurance claim processing centers, and create automated patient entrance forms.
It also has a mobile app for iOS users, can accept data input from electronic optometry equipment, and can be integrated with different applications.
ManagementPlus

Last but not least, ManagementPlus practice management software for optometrists was designed as a fully-fledged and customizable solution with a bunch of functions. With the help of this soft, eye care specialists can work with EHR, PM, ASC forms and inventory. It is also quite helpful in managing revenue cycle services, practice building and reputation management, business analytics and capital funding.
What is more, ManagementPlus solutions allow optometrists and clinic managers to work in one platform, which makes communication clear and unified. Users can track workflows and handle all billing from eligibility to collections.
In addition, ManagementPlus has an in-built reporting tool, which allows specialists to report on most fields in the system, while the practice management system provides a choice of two scheduling modules. Users have the option of choosing either cloud-based or on-premise deployment.
Summing up
Optometry management software is a perfect choice for any medical practice, including solo practices, midsize clinics, and large hospitals. It is a perfect tool not only for managing patients, optical instruments and aids. The software is also helpful in improving operations, increasing efficiency and revenue and streamlining the working process. Such solutions keep all the data in one place, powering optometrists to document the patient history directly from diagnosis, and managers to avoid unnecessary paperwork.

AI for OCT scan analysis
Register in a free Demo Account to see how it works: OCT scans with AMD, DR, early glaucoma are already inside.
Overall, optometry management software is a need for modern practice, as it improves the diagnosis and treatment, and even can be integrated with image management systems, like Altris AI. This integration assists in managing patient data, helps with controversial OCT scans, differentiate between pathological and non-pathological scans, and, most importantly, gives confidence to eye care specialists.

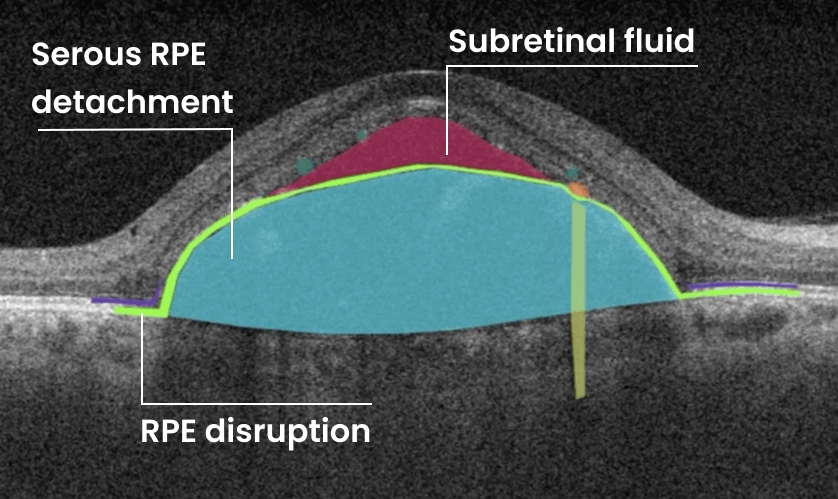










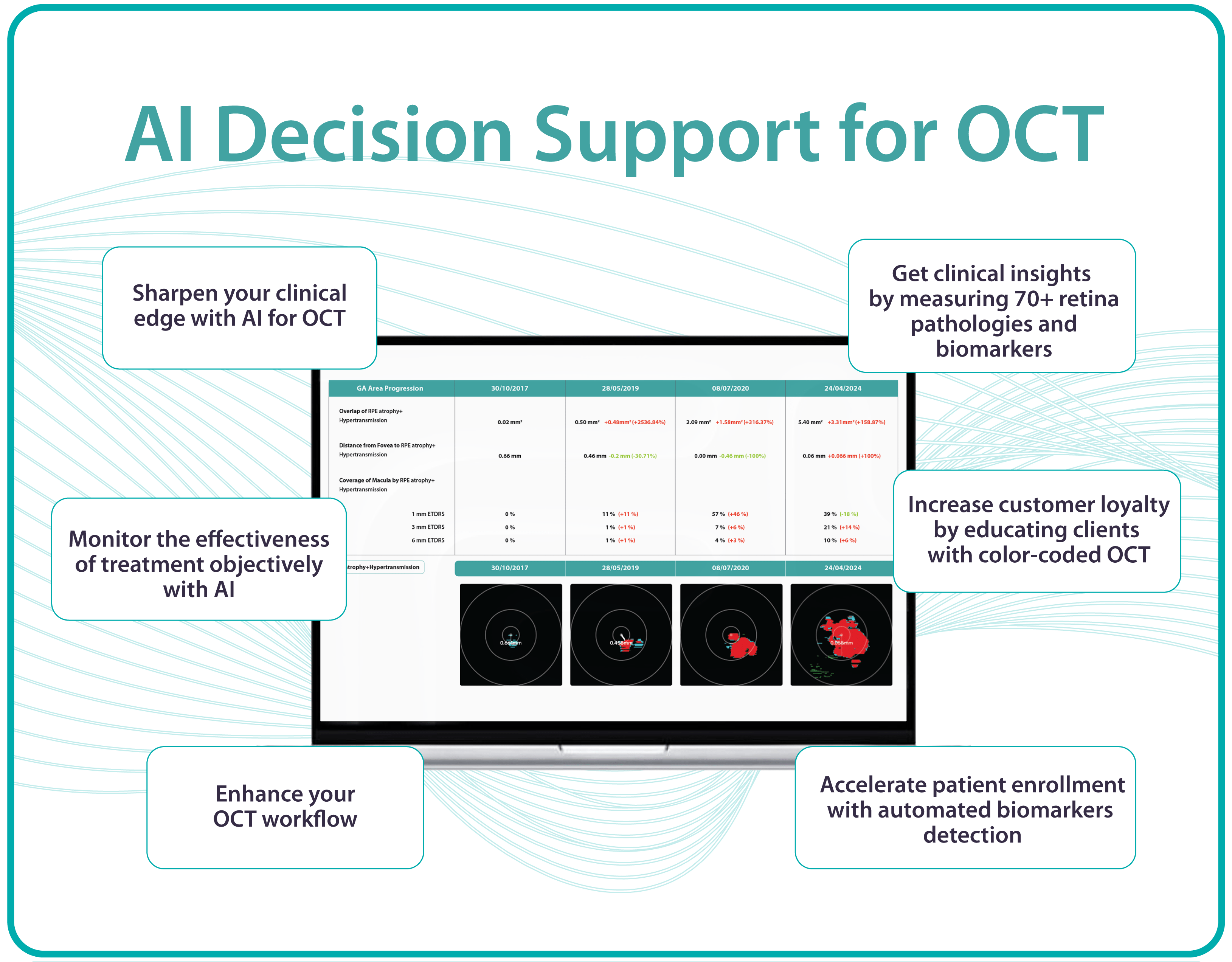

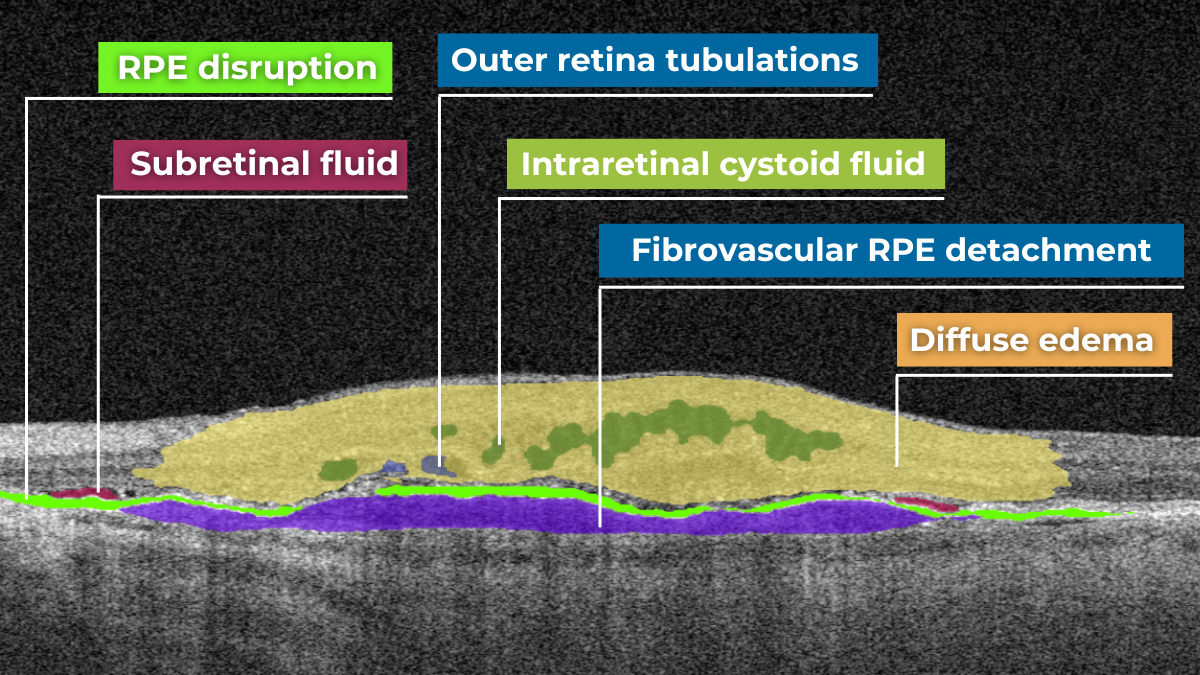
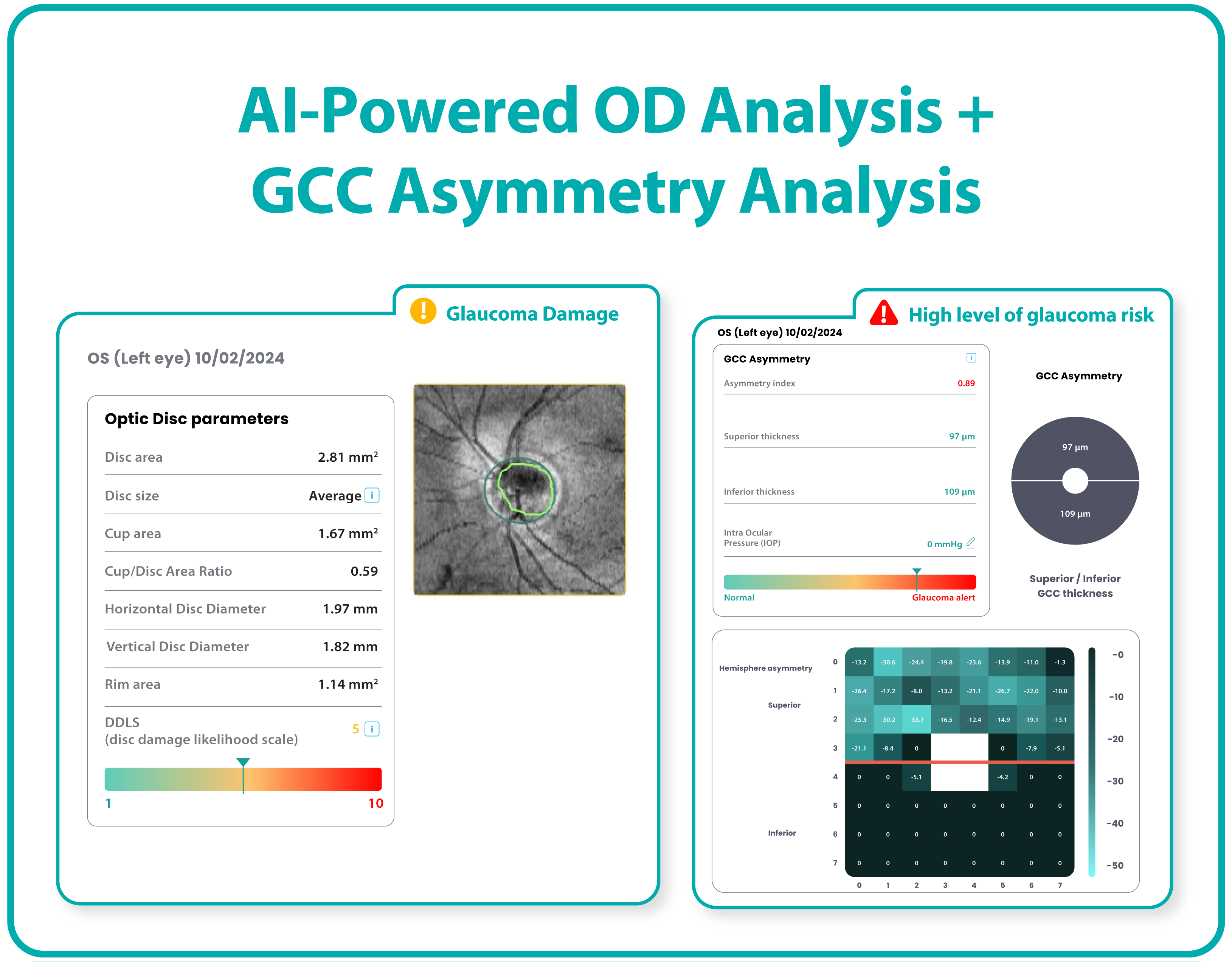



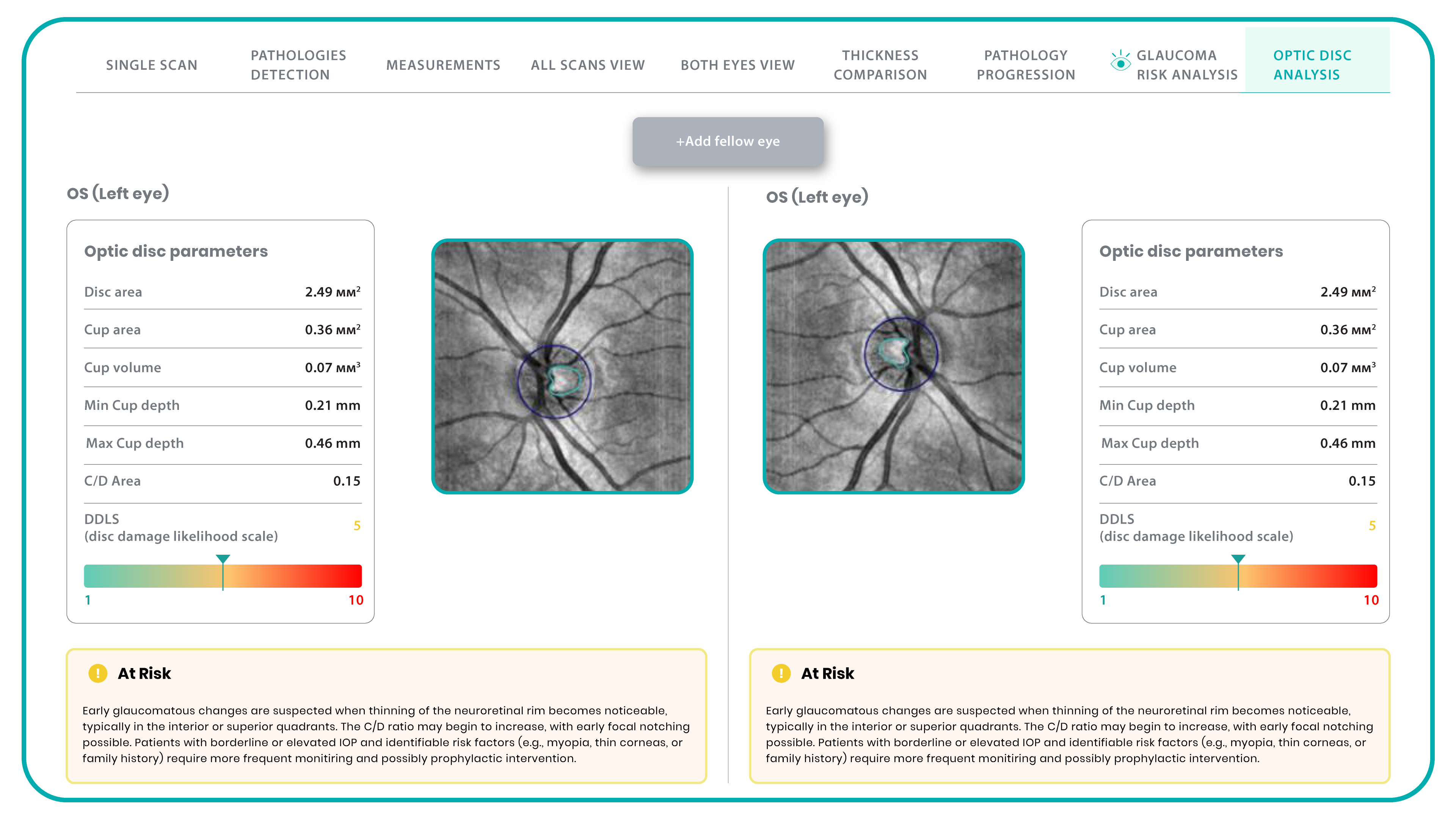










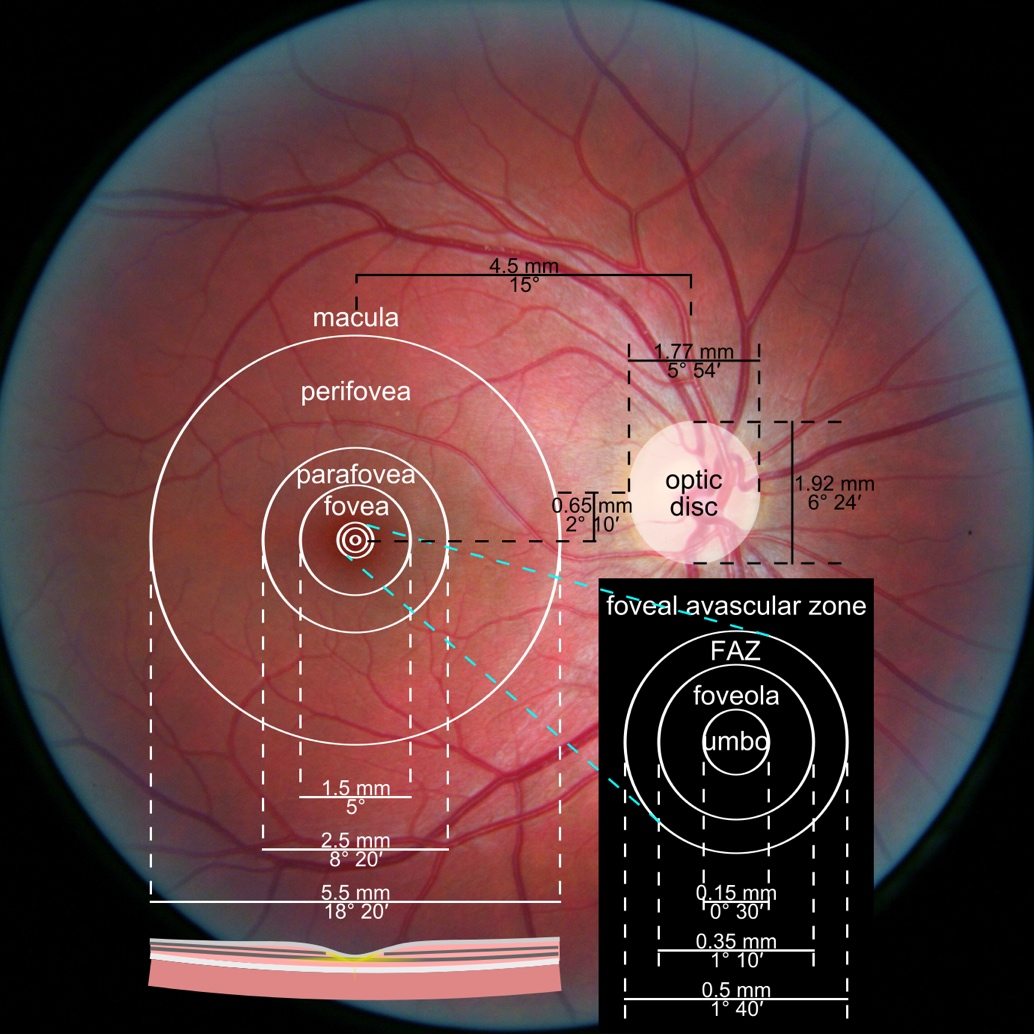
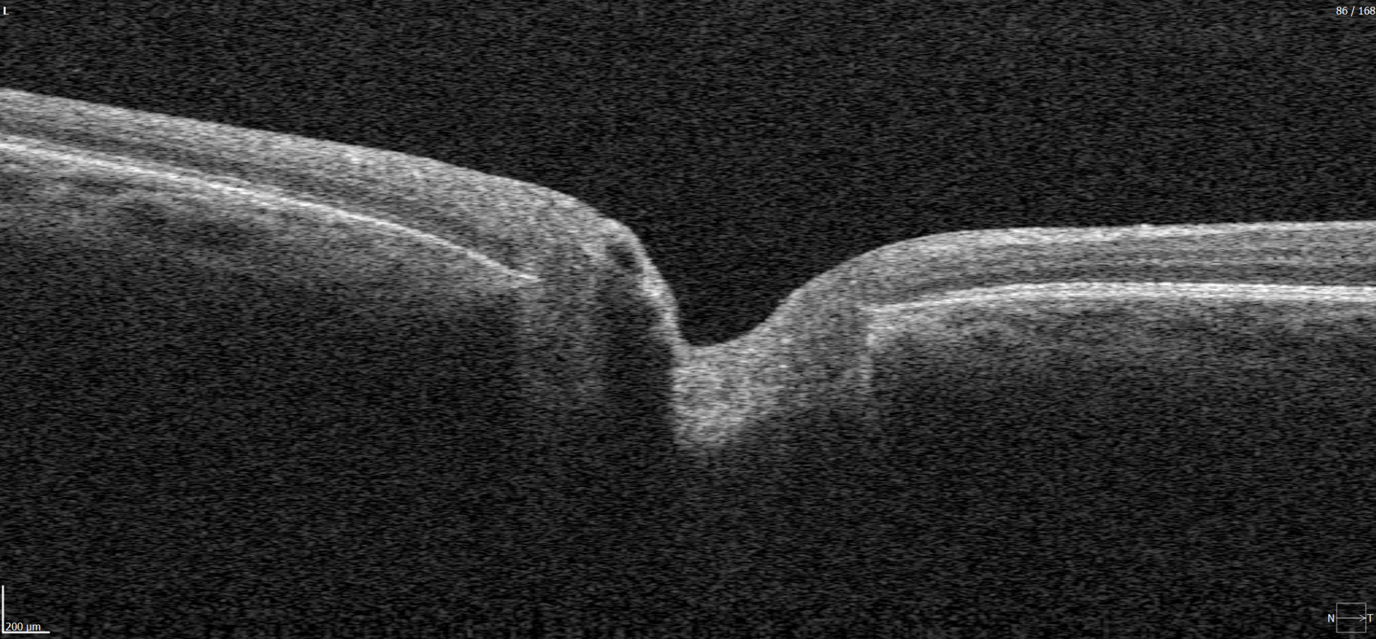
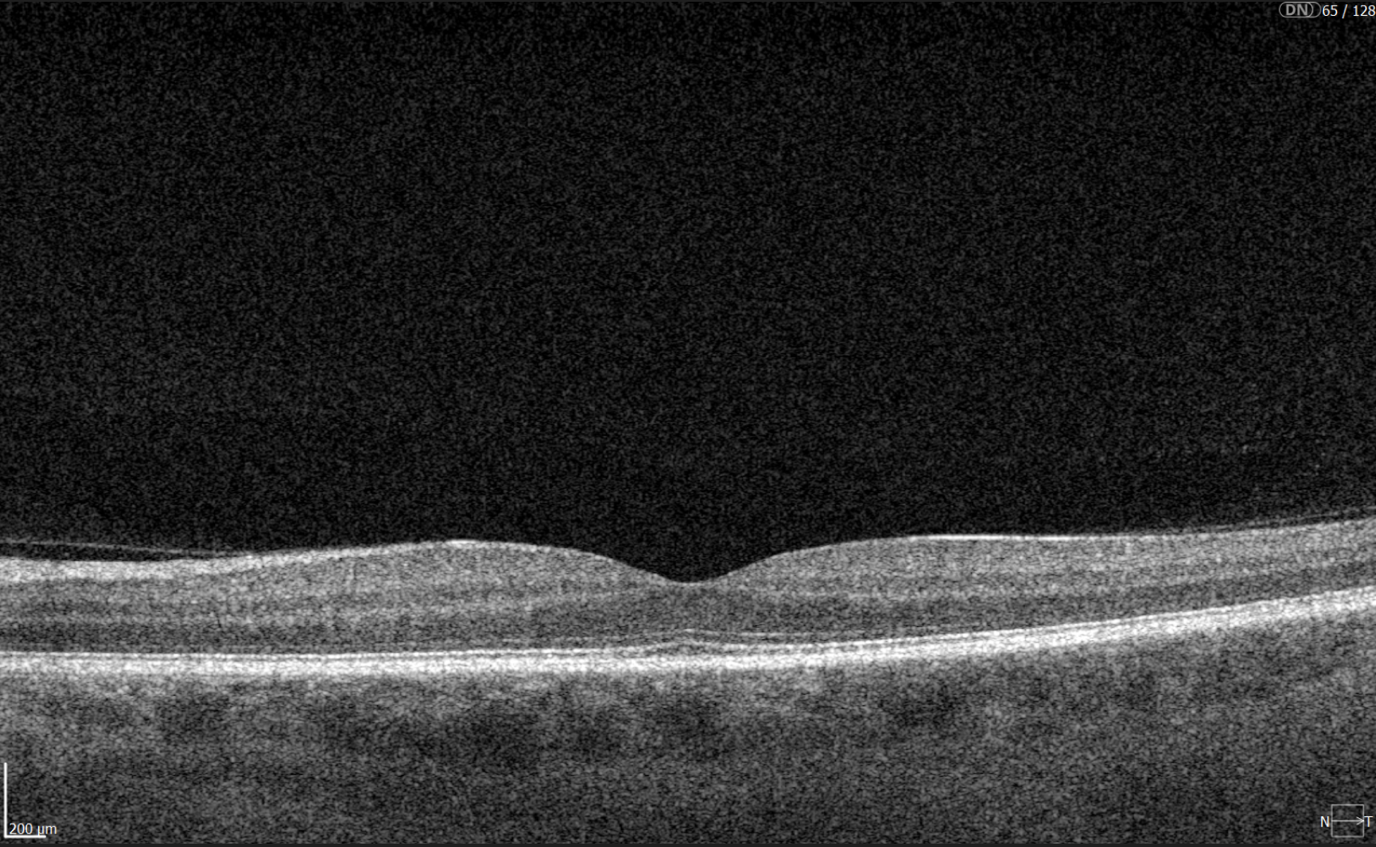
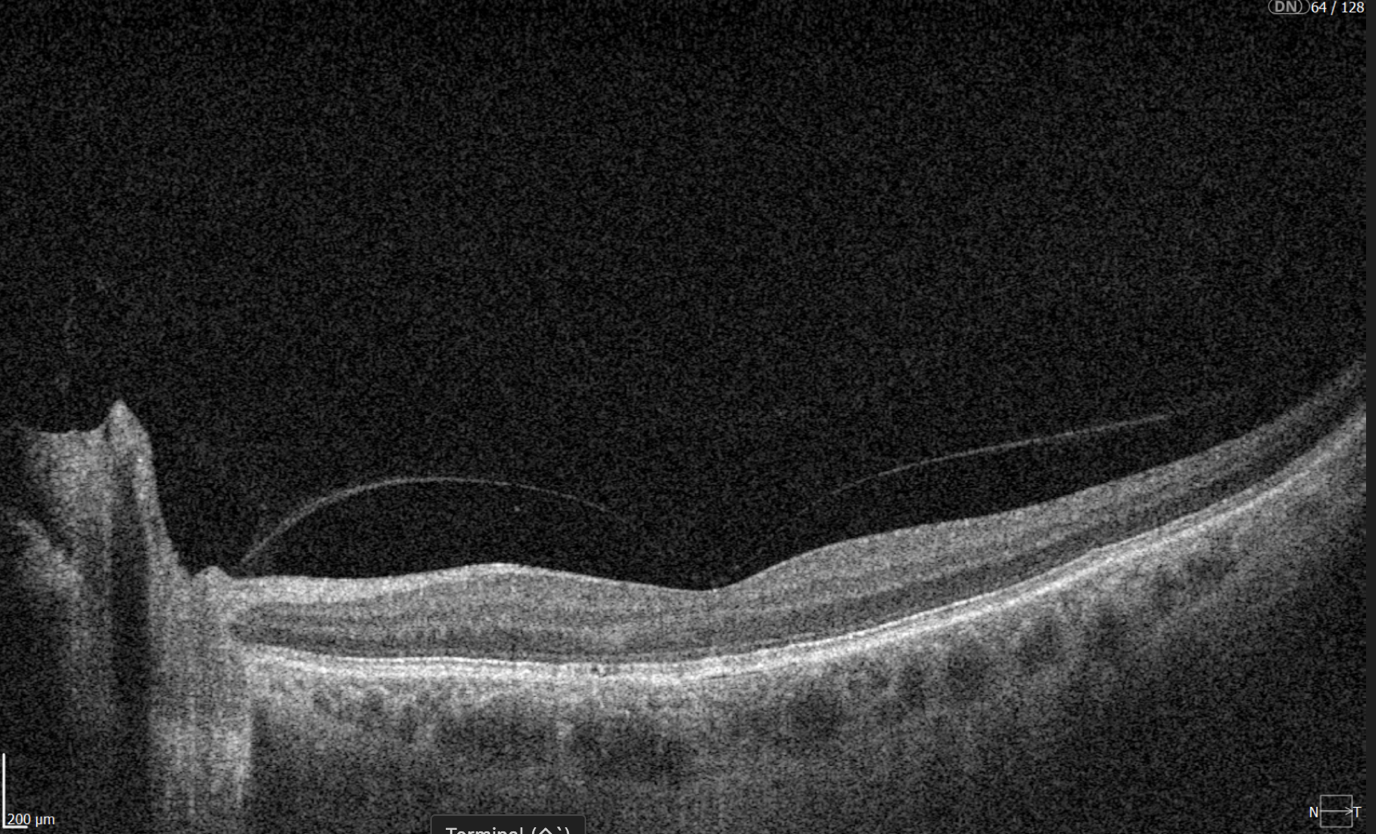
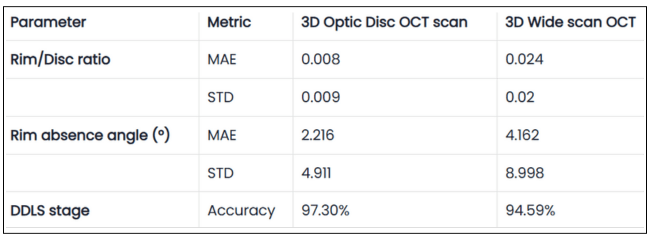
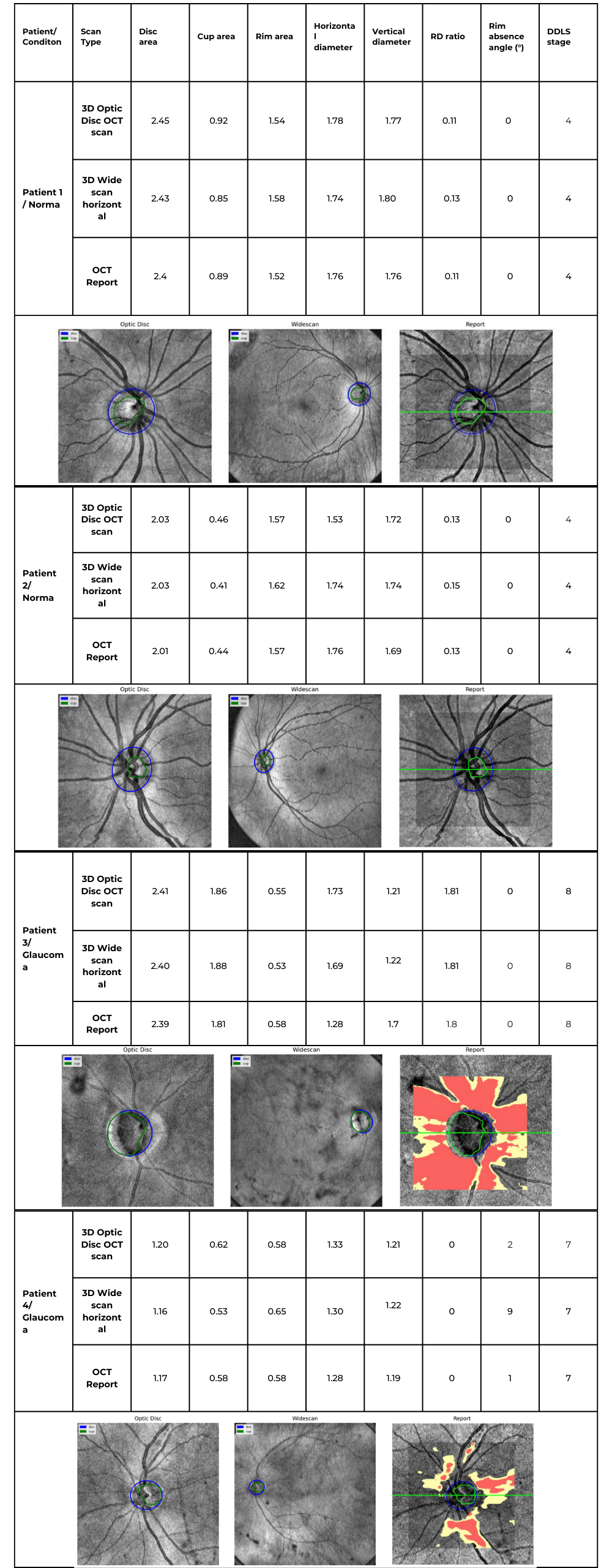
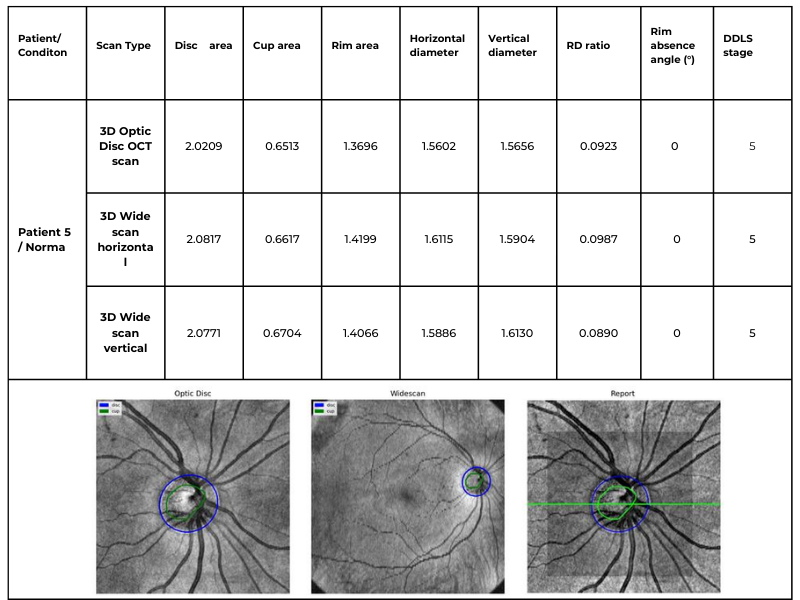
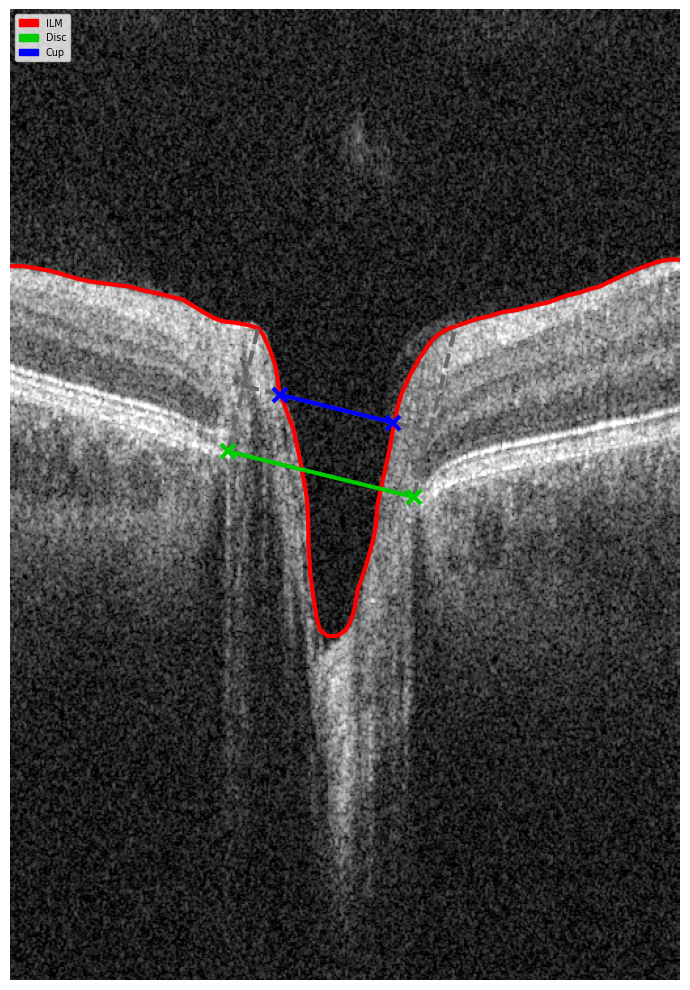
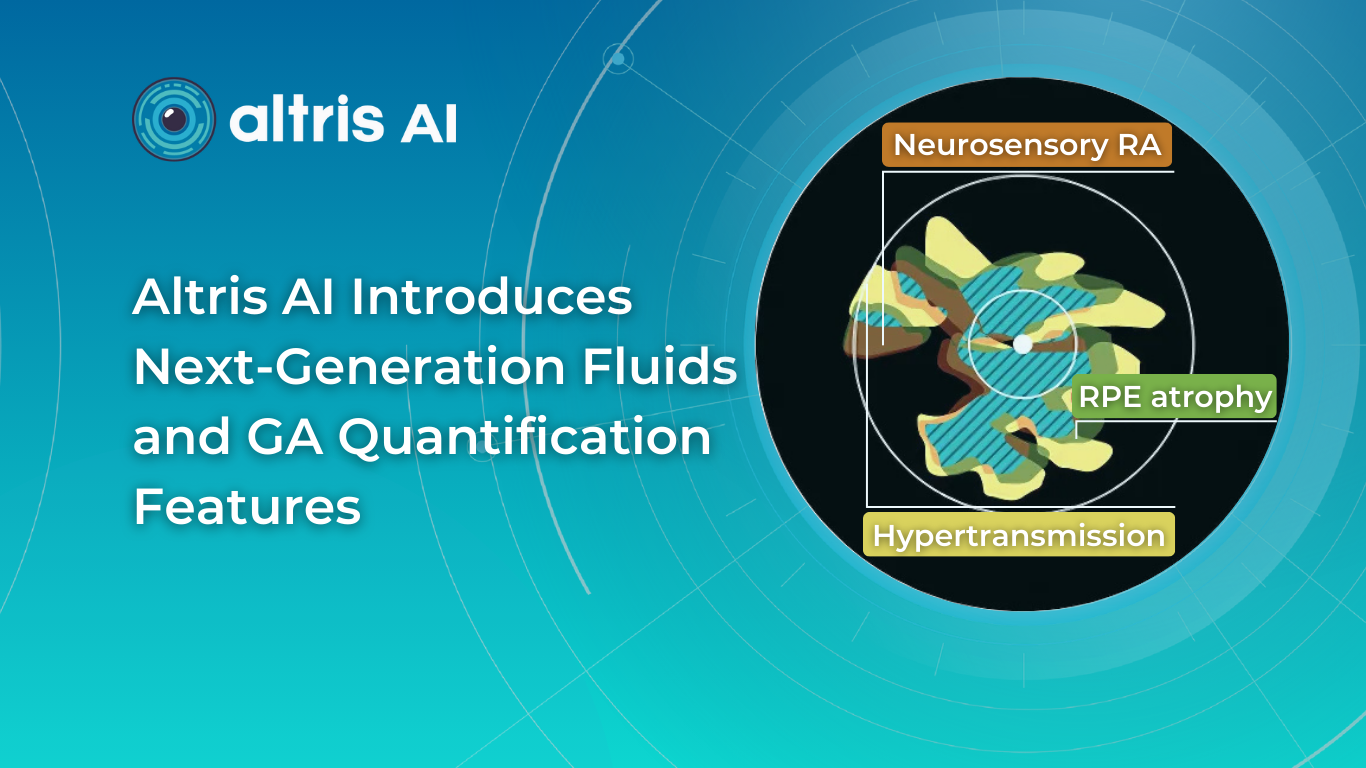
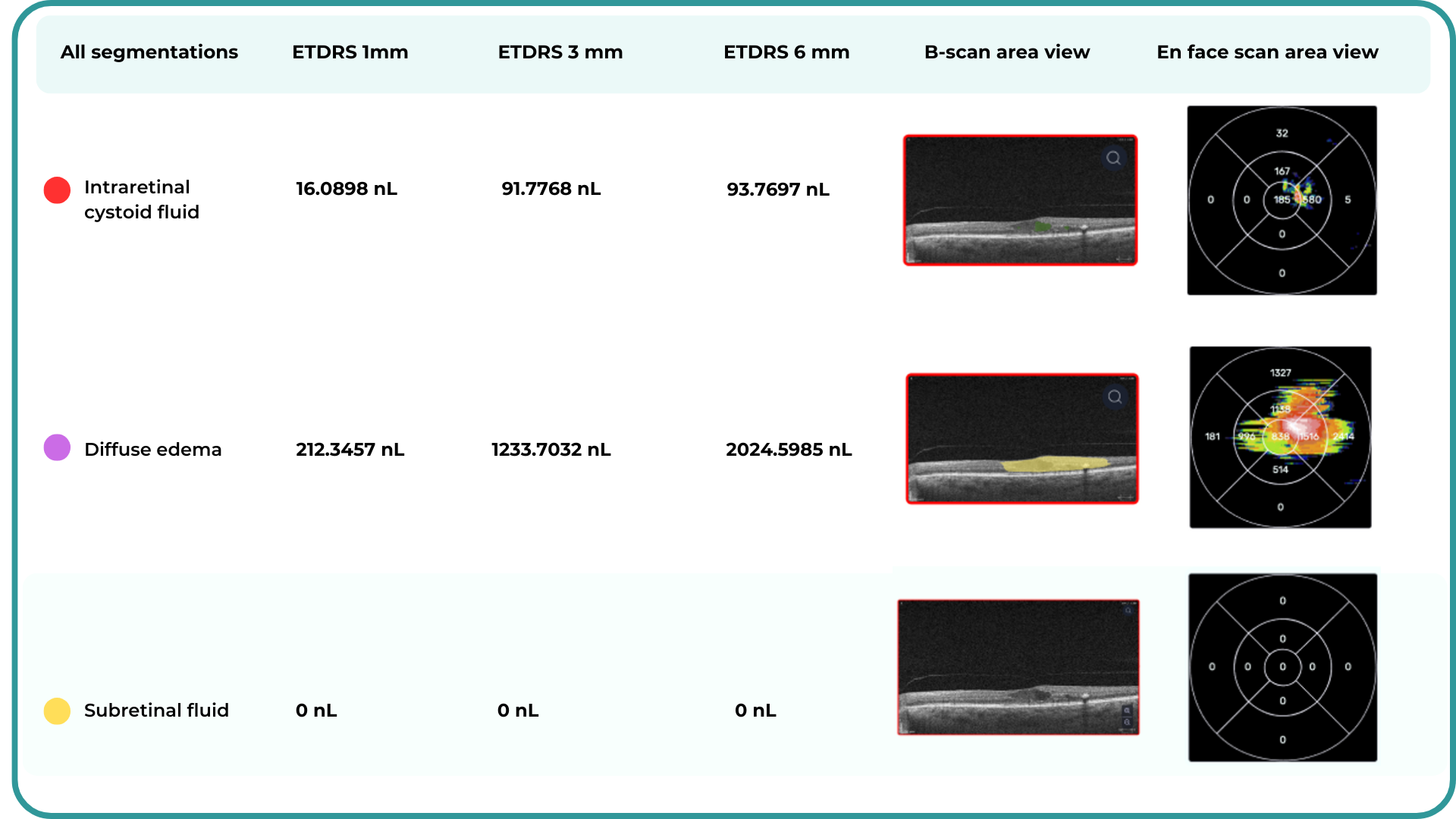
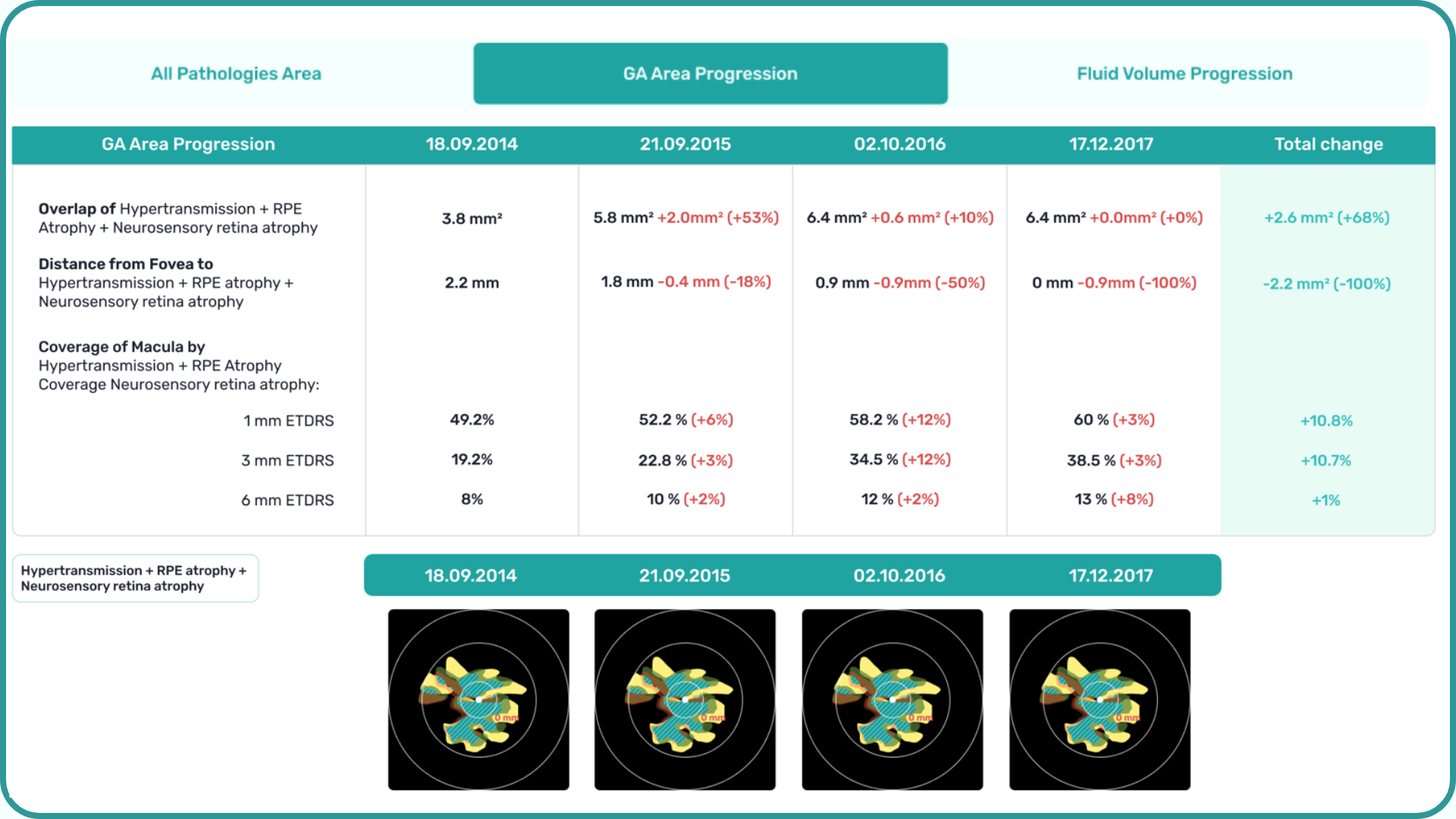
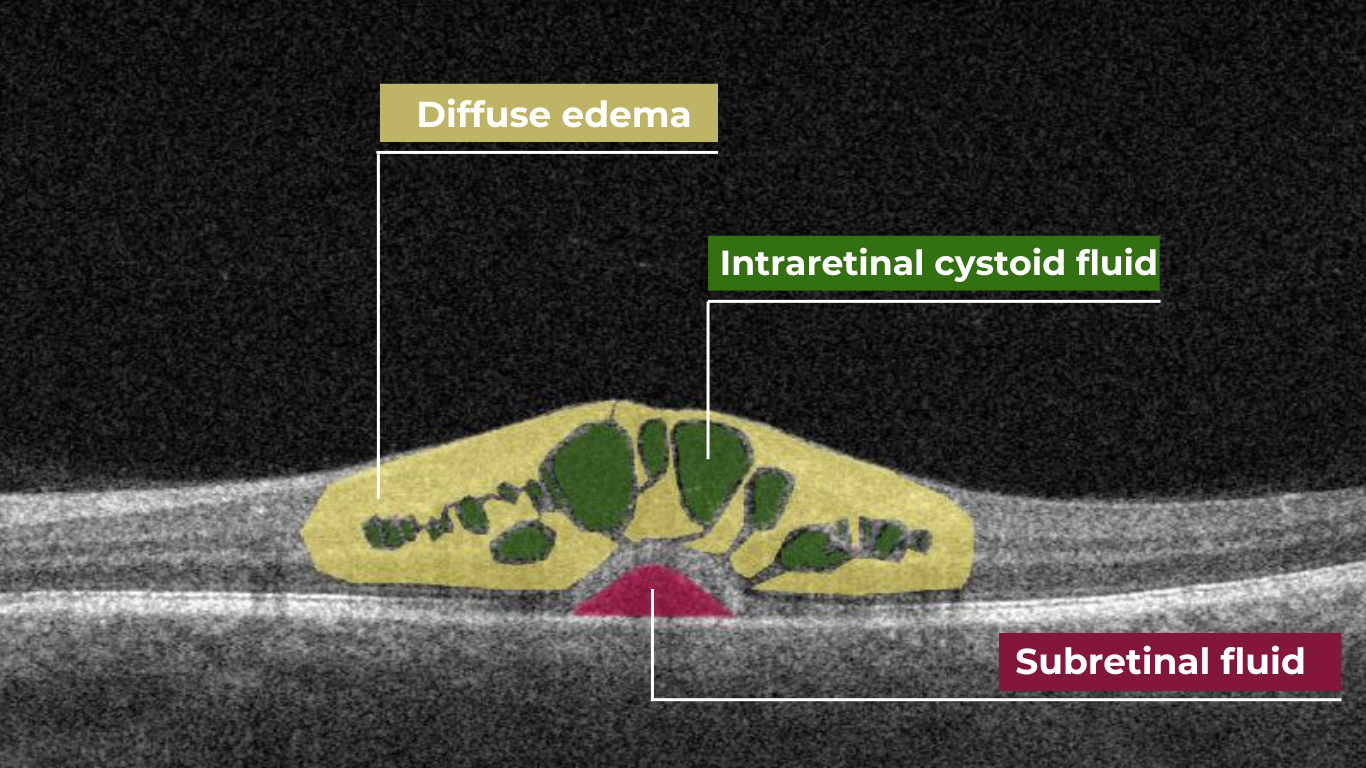
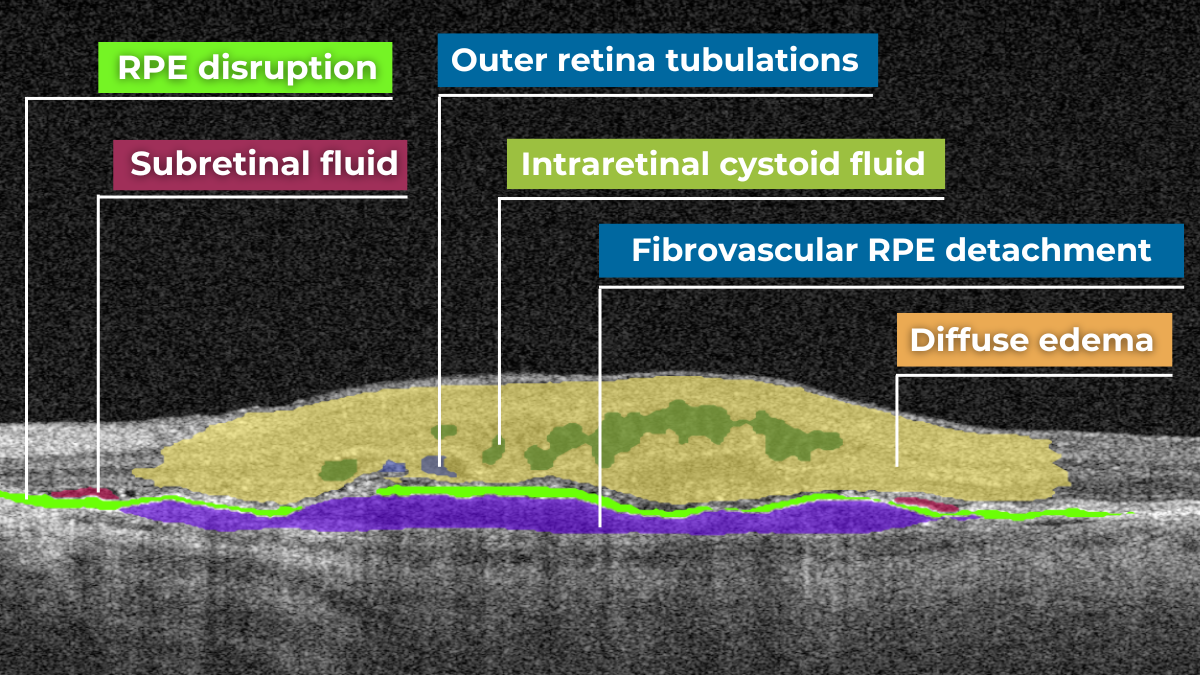
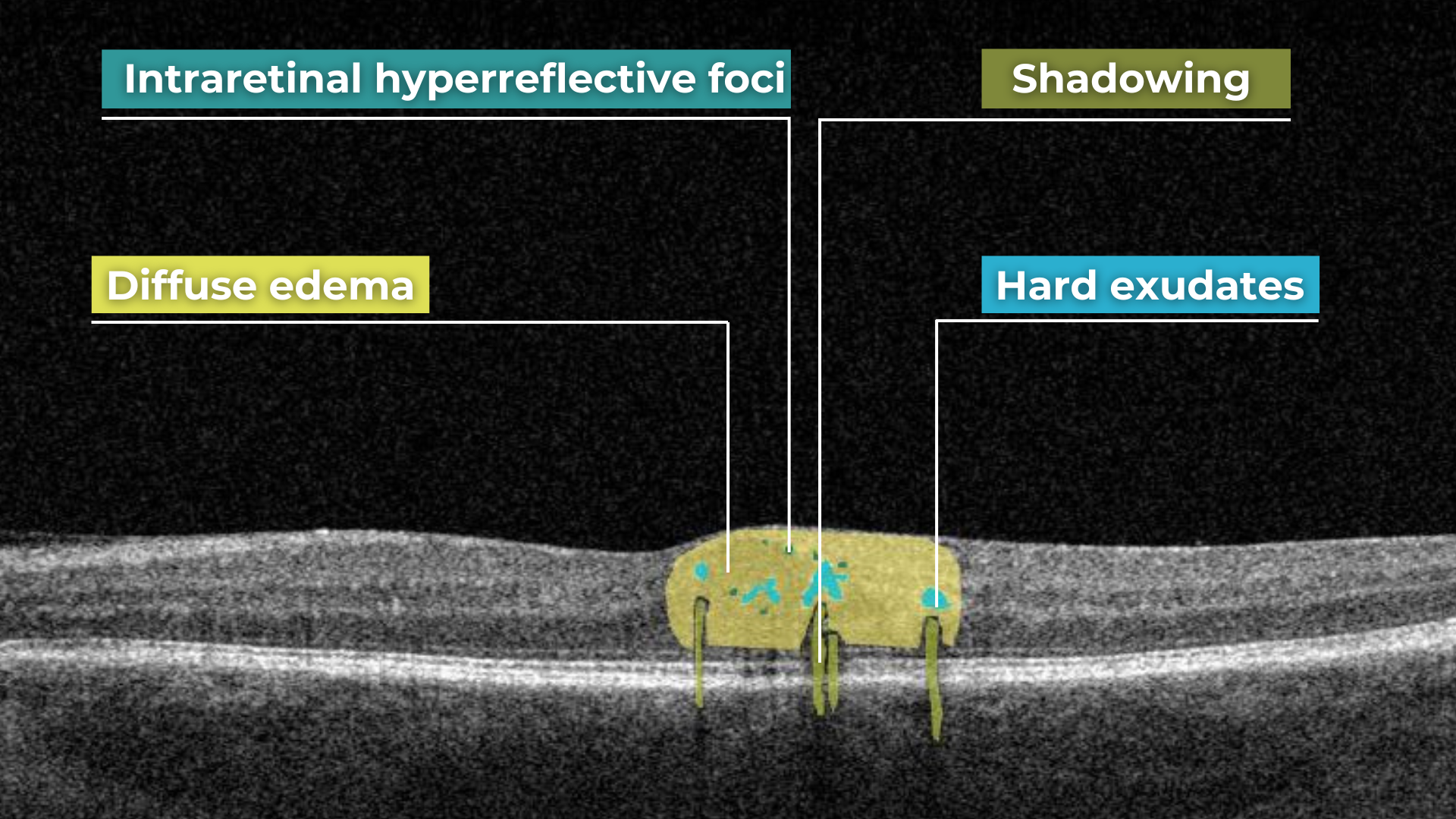
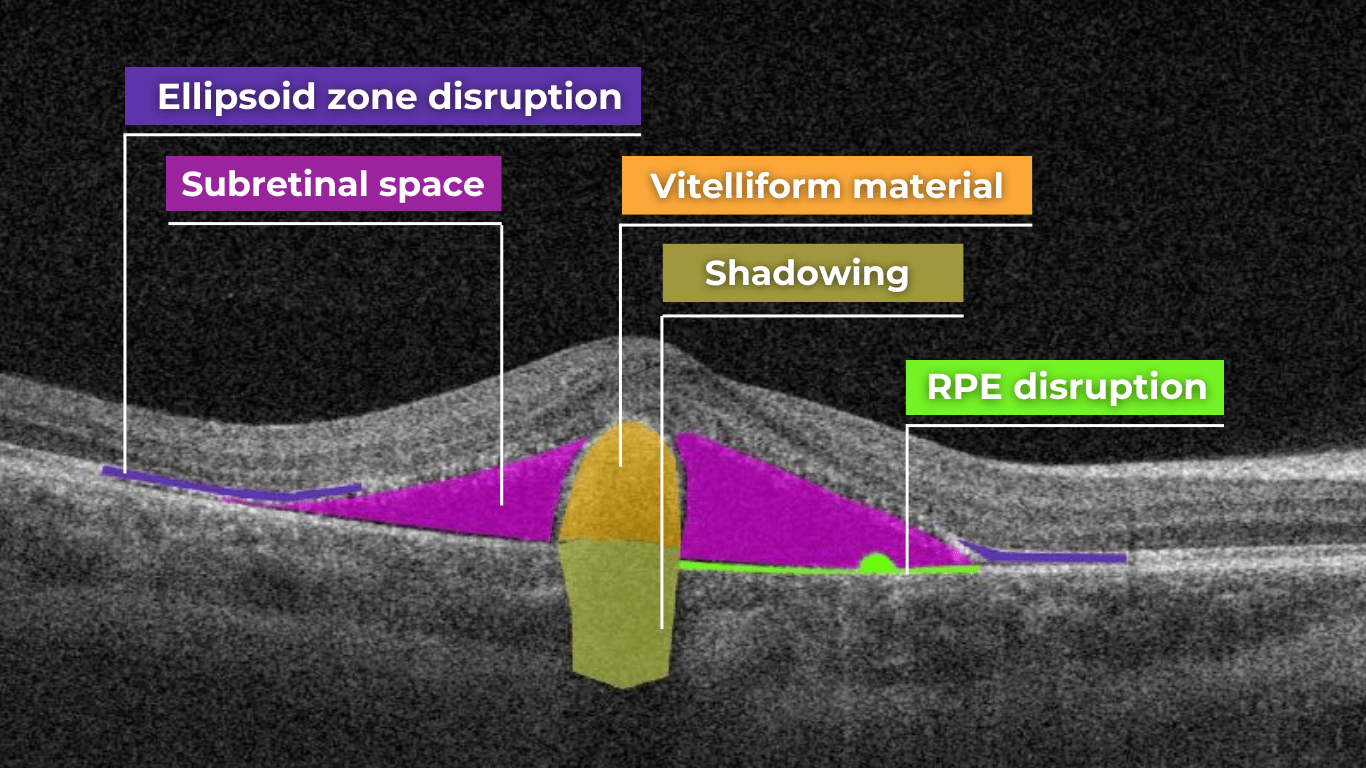
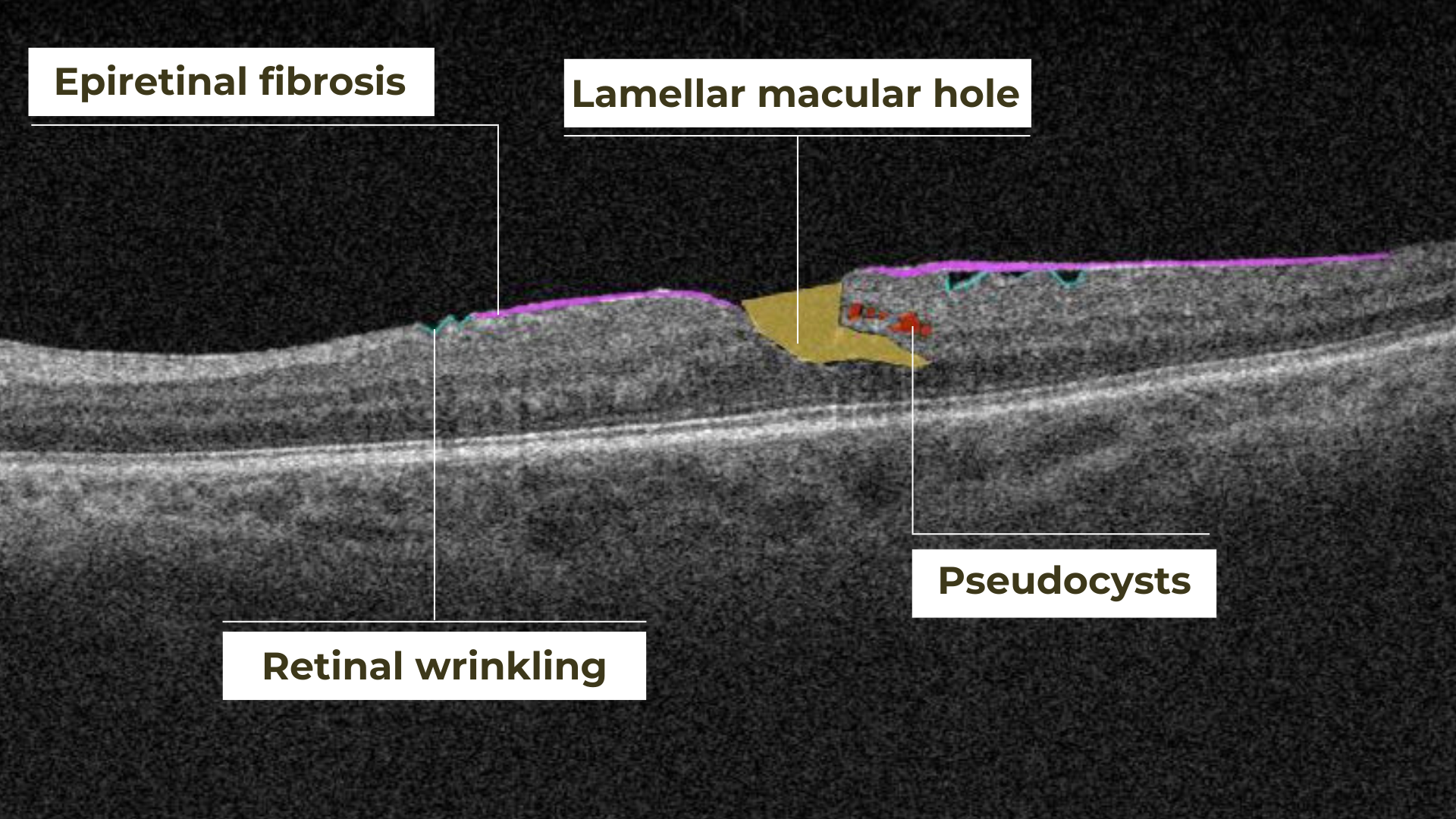
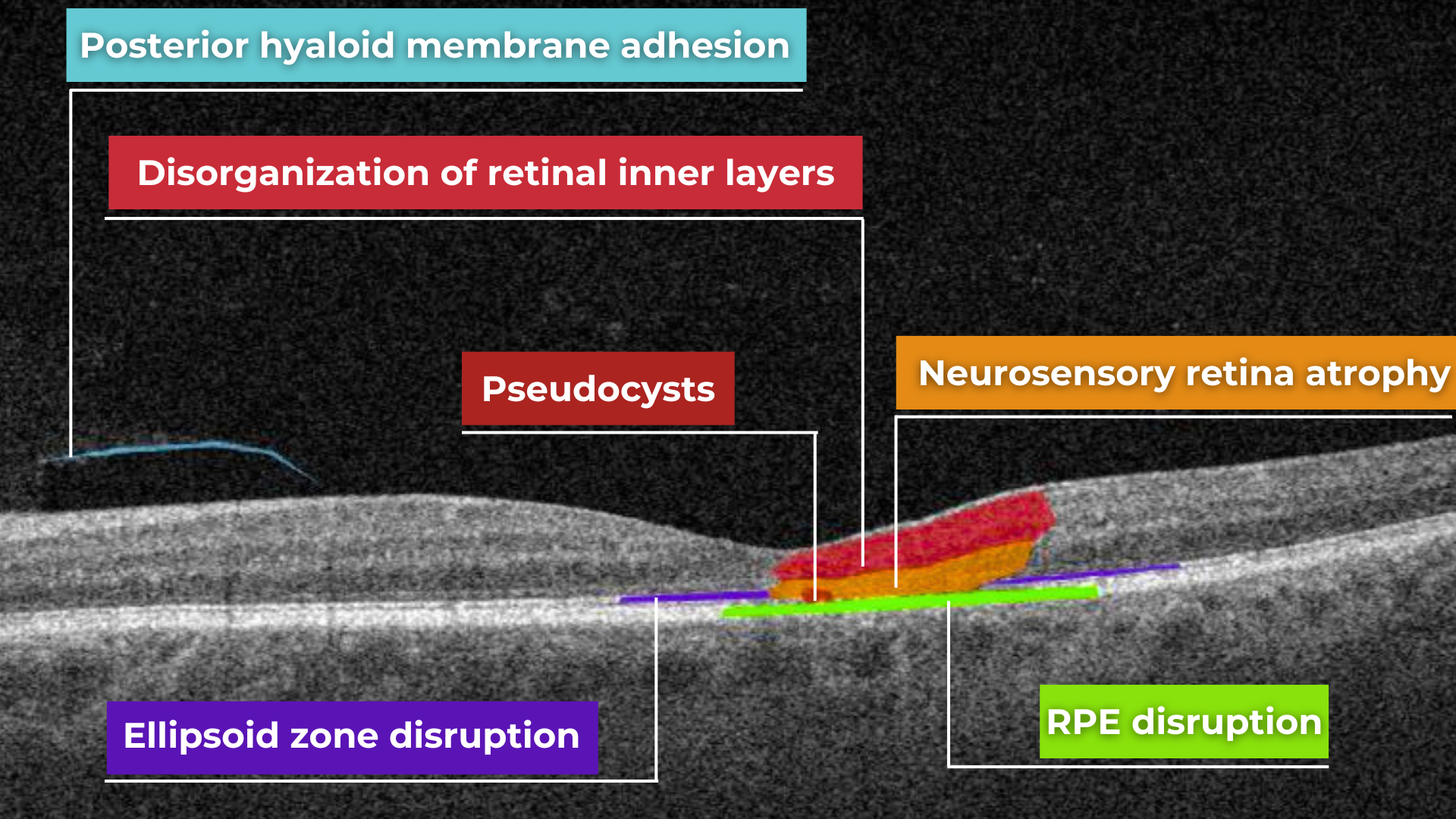
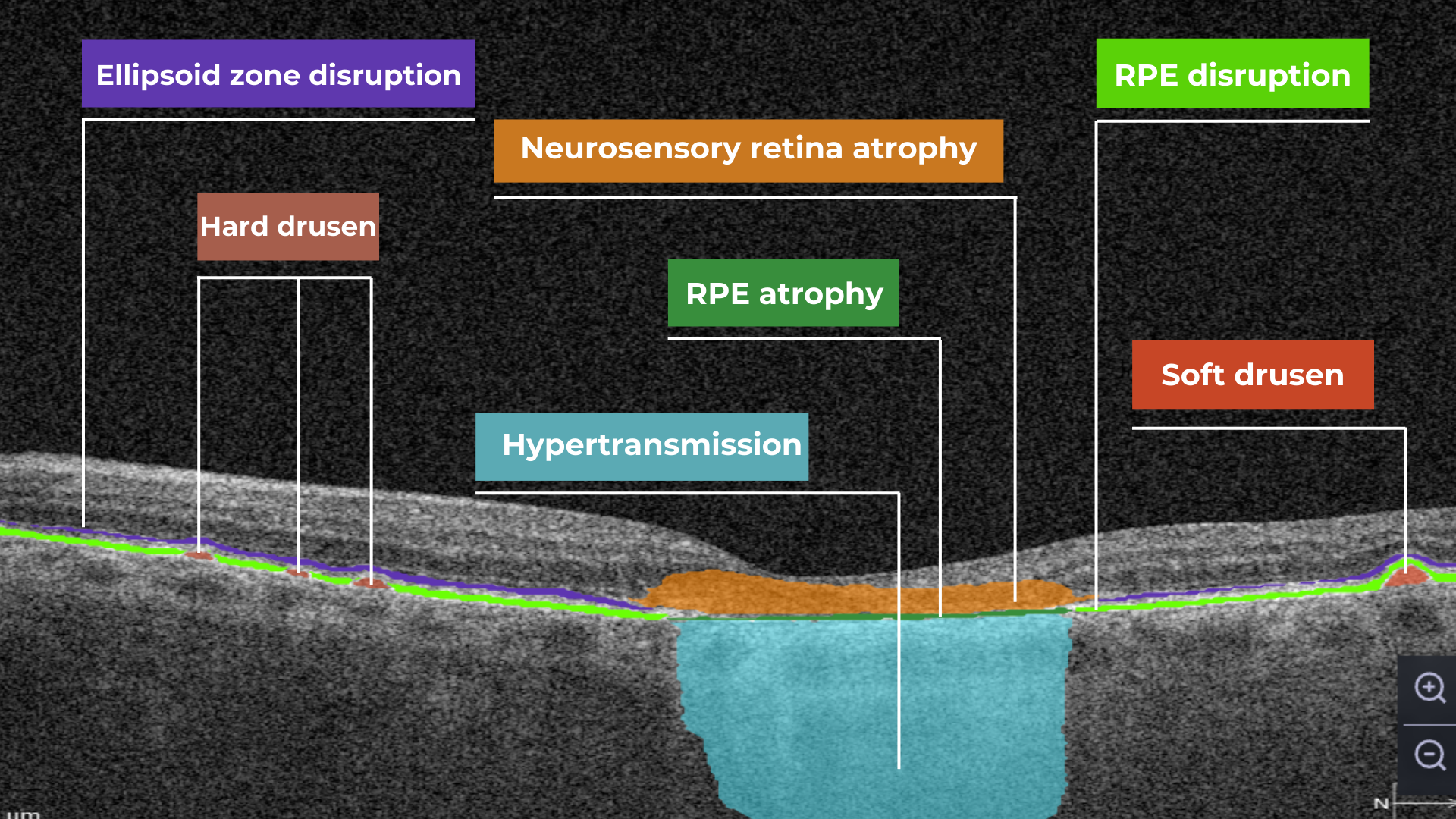
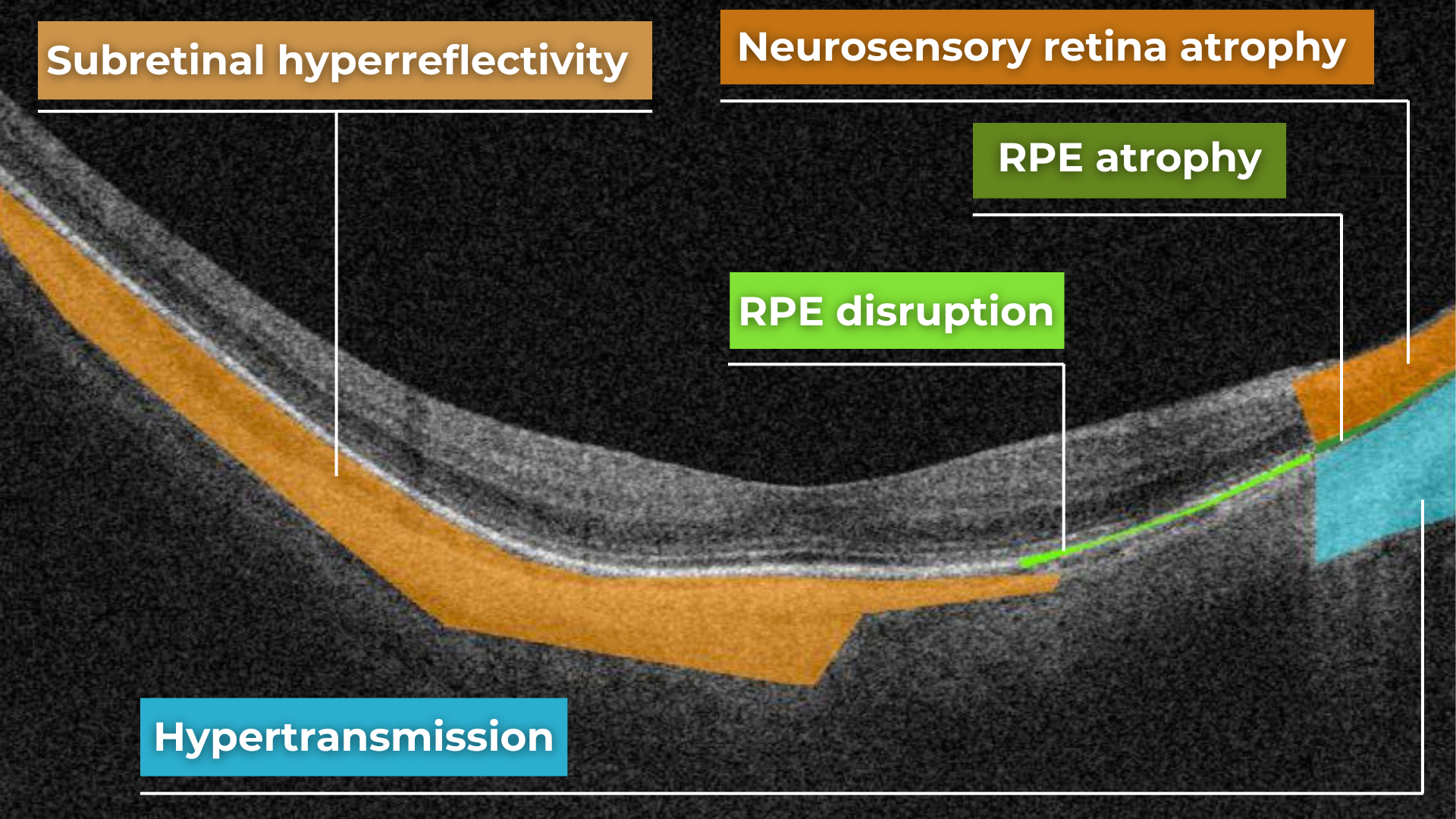
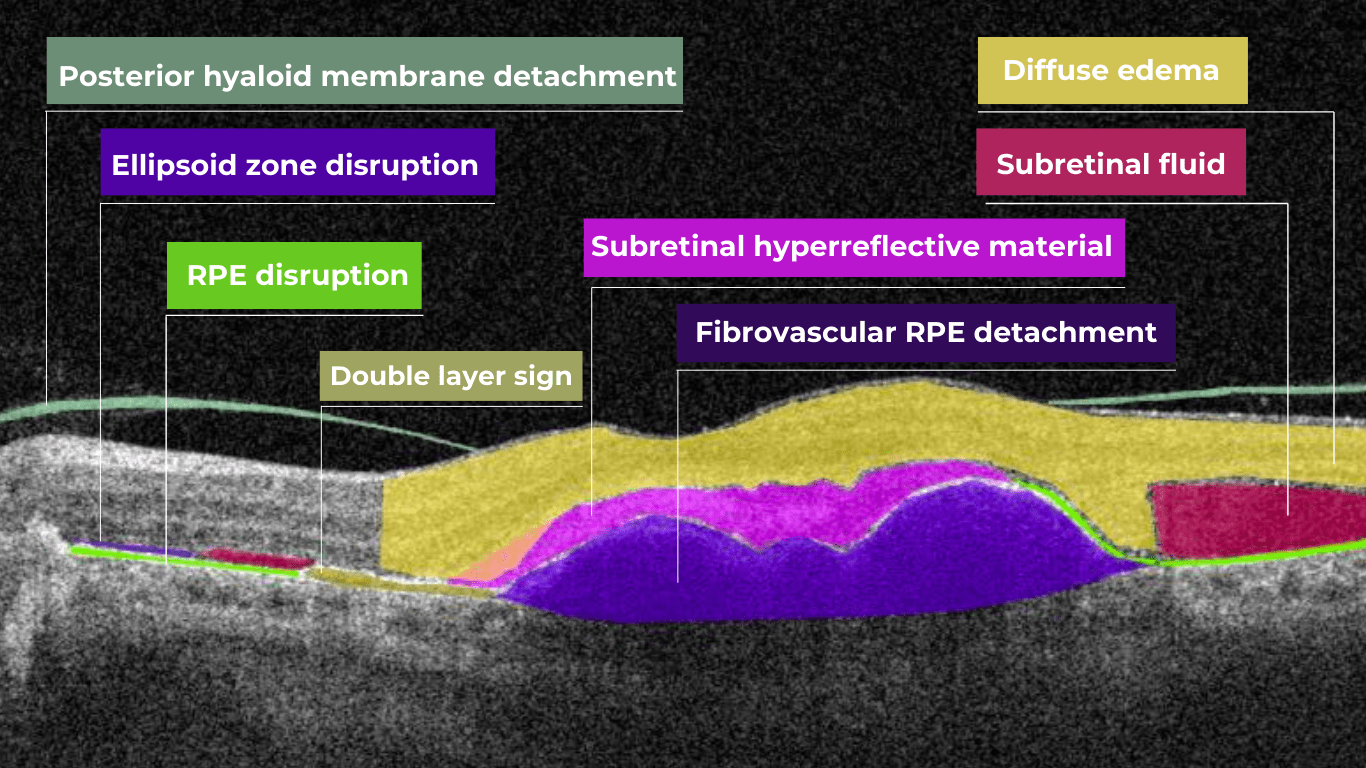
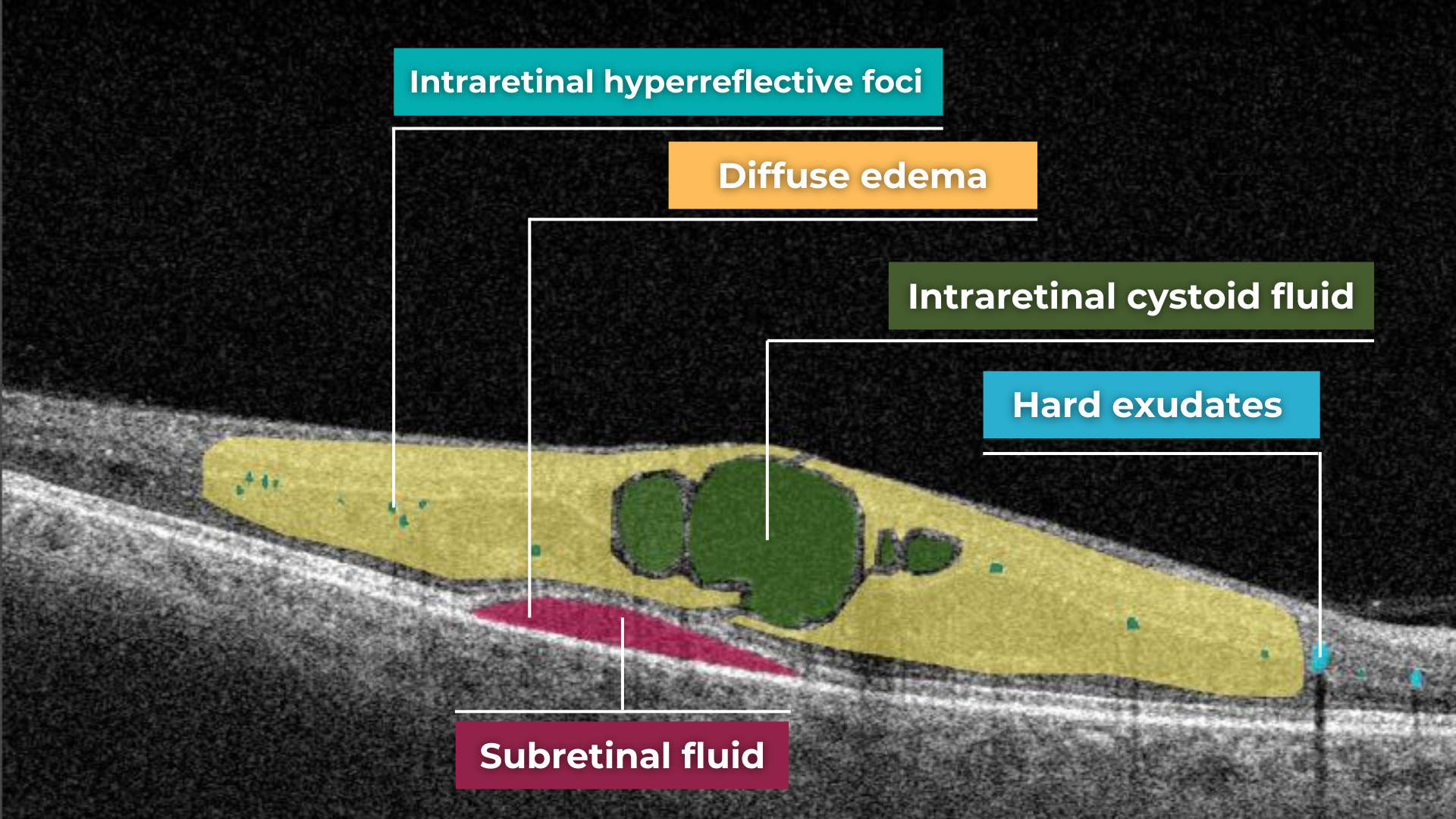
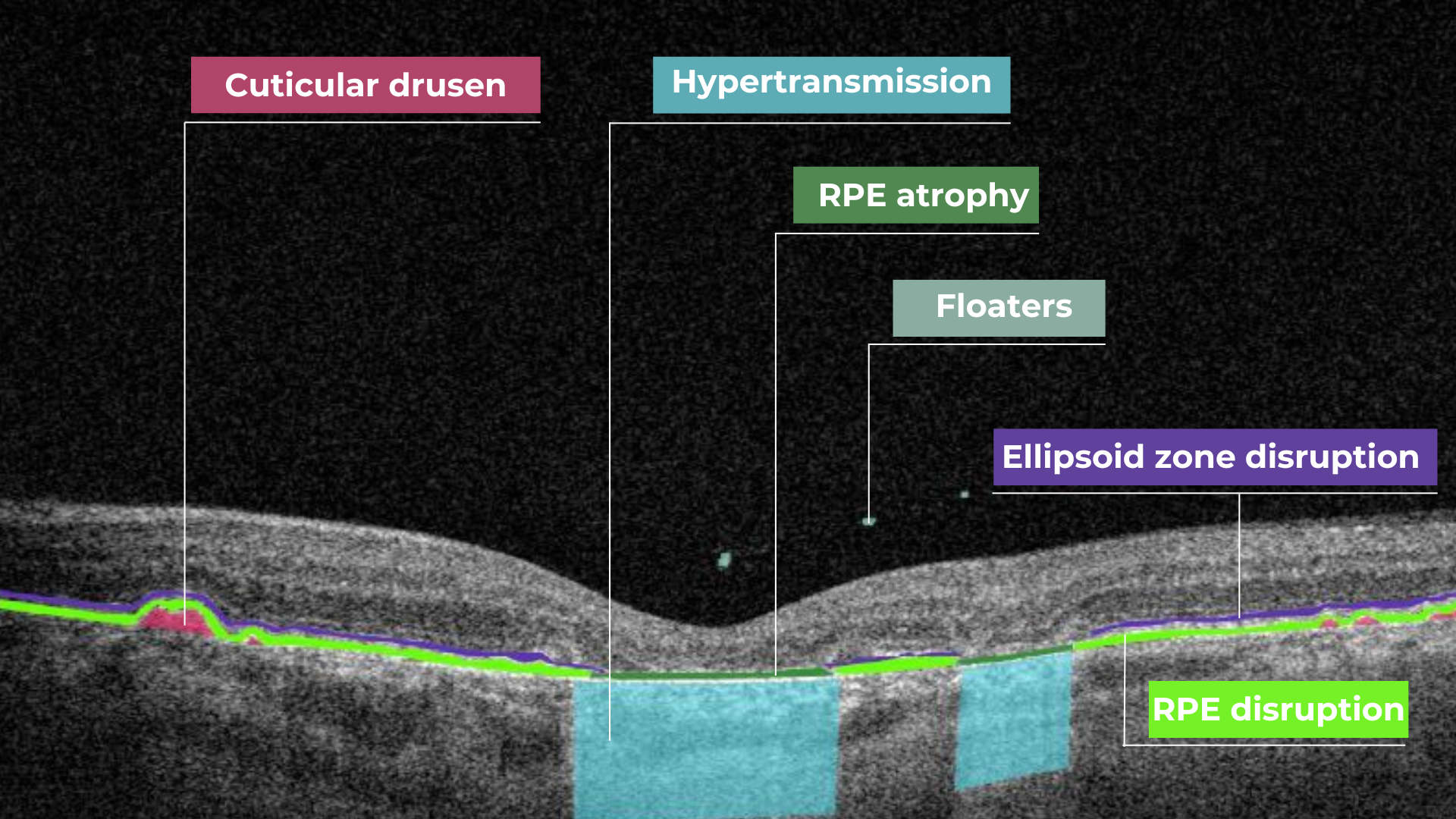 Hypertransmission on OCT
Hypertransmission on OCT