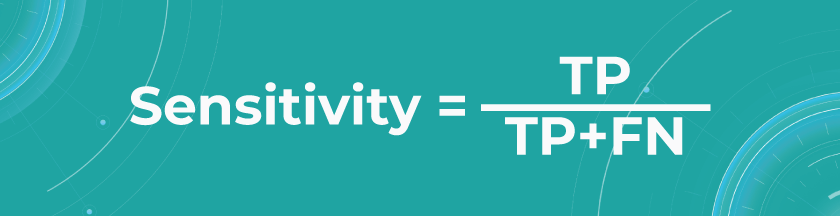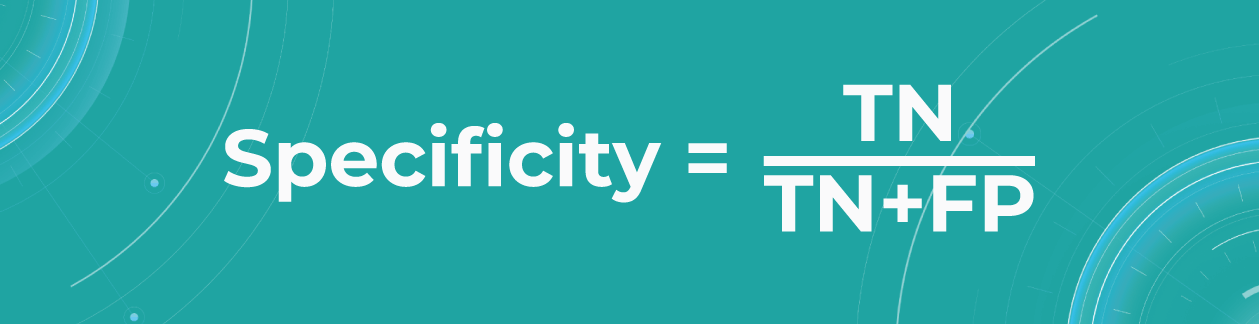Recently Posted
-

Eye Hospital Management Software: Top 8 Solutions for your Clinic
 Maria Znamenska
04.01.202310 min read
Maria Znamenska
04.01.202310 min readThe term “Eye hospital management software” can have numerous meanings. Some soft can be a part of larger EMR (electronic medical records) systems, some can help with scheduling and billing, and some can help with patients’ information management. There is also an eye clinic management system that can even advise on diagnosis based on the patient’s history and medical images. Because of dozens of different soft on the market, it can be quite complicated to choose a proper set of tools for your practice.
If you are an ophthalmologist or manage an ophthalmic diagnostic center/hospital, you may have trouble choosing the right software. That is why we’ve decided to prepare a list of solutions for patients’ health recording and diagnosis. We will highlight the benefits of the ophthalmic practice management system and help you choose the right solution.

AI Decision Support for OCT
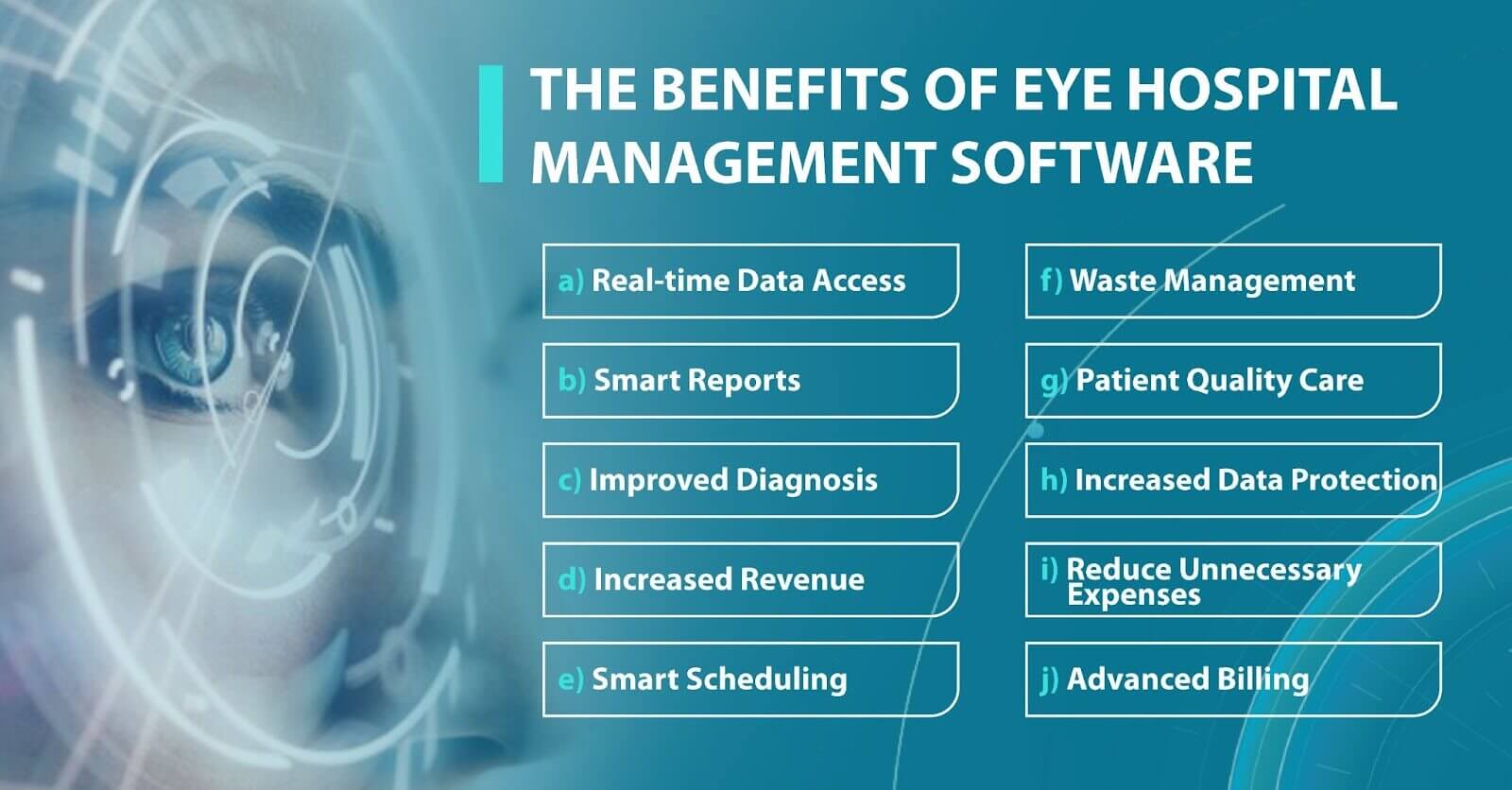
Why eye hospital management software is worth using
Eye hospital management software has become extremely important for eye clinics or medical centers looking to streamline their workflows, automate processes, and provide higher-quality care with less effort. You can have piles of paper and numerous excels, but when someone is on vacation, it will be impossible to make sense of all data and use it quickly.
However, many clinics still work according to the old scheme and refuse to introduce new technologies into their clinical practice. There may be several reasons for this: mistrust of modern tools, reluctance to spend the money buying licensed eye clinic management system, or reluctance to spend staff time learning how to work with the program. But, in fact, today, there are systems designed specifically for ophthalmologists to function flawlessly in eye care settings. Here are some benefits that an eye clinic management system can provide to your medical practice. Let’s take a closer look at some of them:

- High level of data protection. Another important benefit of the ophthalmic practice management system is a high level of data protection. High-quality soft gives access to data only to authorized persons. The software also has security systems that guarantee no risk of data loss and full protection of medical history or information about the patient’s condition.
- Increasing diagnostic accuracy. Using an eye clinic management system, ophthalmologists improve the quality of diagnosis and treatment, as they get access to the whole patient’s history from the past to the present. An ophthalmologist can learn about the previous treatment their patient received and about chronic illnesses. By learning this, doctors can create a better treatment plan.
- Increased revenue. Depending on the number of employees in your clinic, you may need dozens to hundreds of personnel to smoothly handle manual processes. And more human resources mean more expenses. However, by using best practice management software for ophthalmology, you can significantly reduce spending and let your employees and doctors focus on the more creative tasks that require empathy and communication.
These are the most common benefits of an eye clinic management system. However, each system has its unique features, so let’s look at the top 8 eye clinic management systems.
Altris AI System

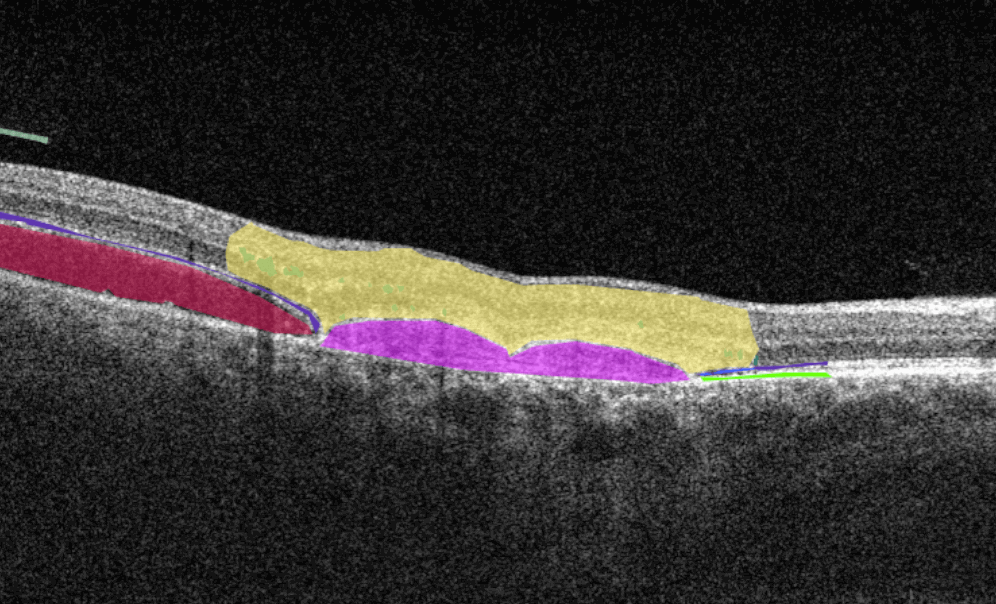
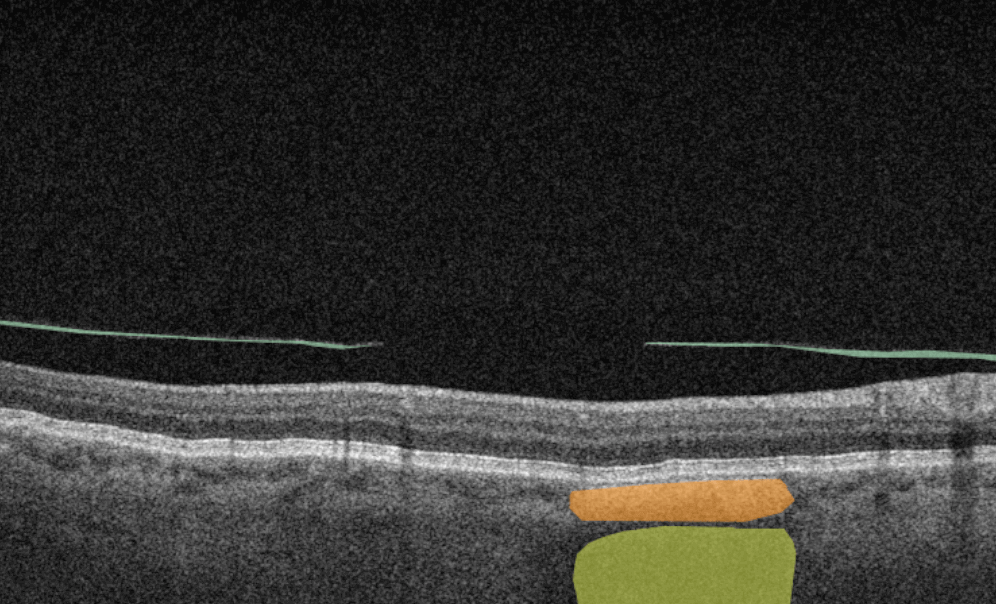
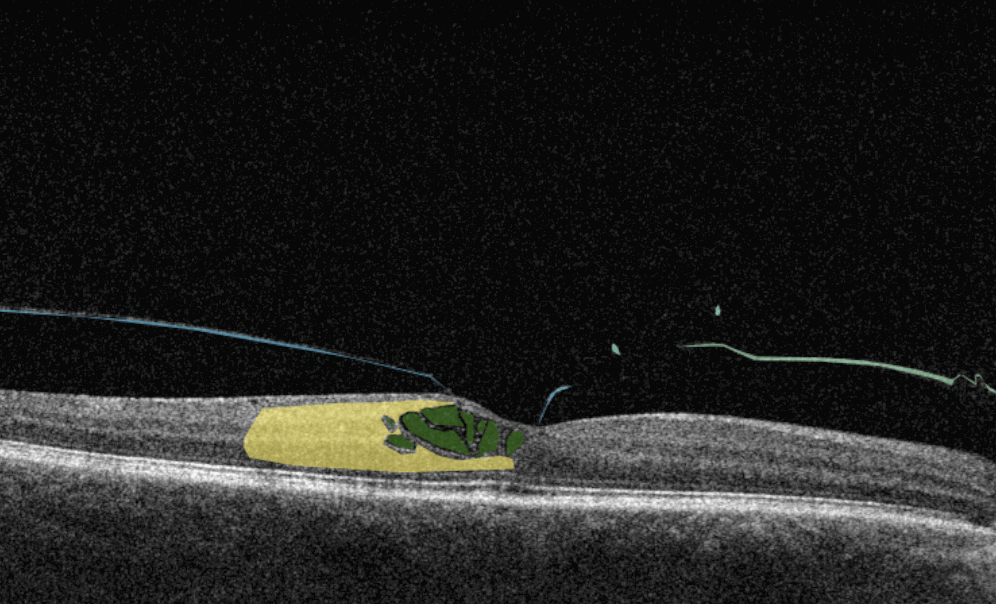
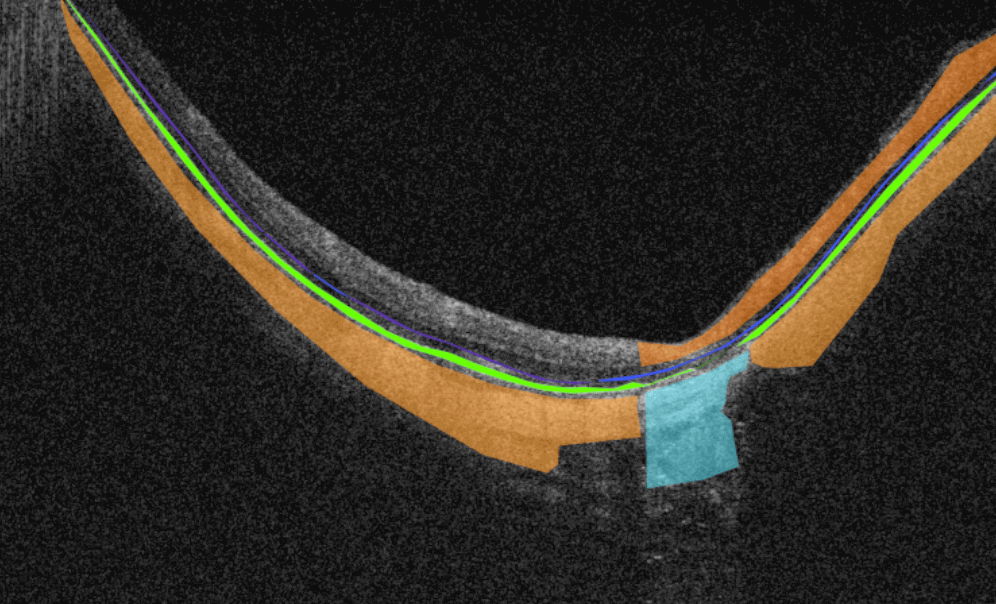
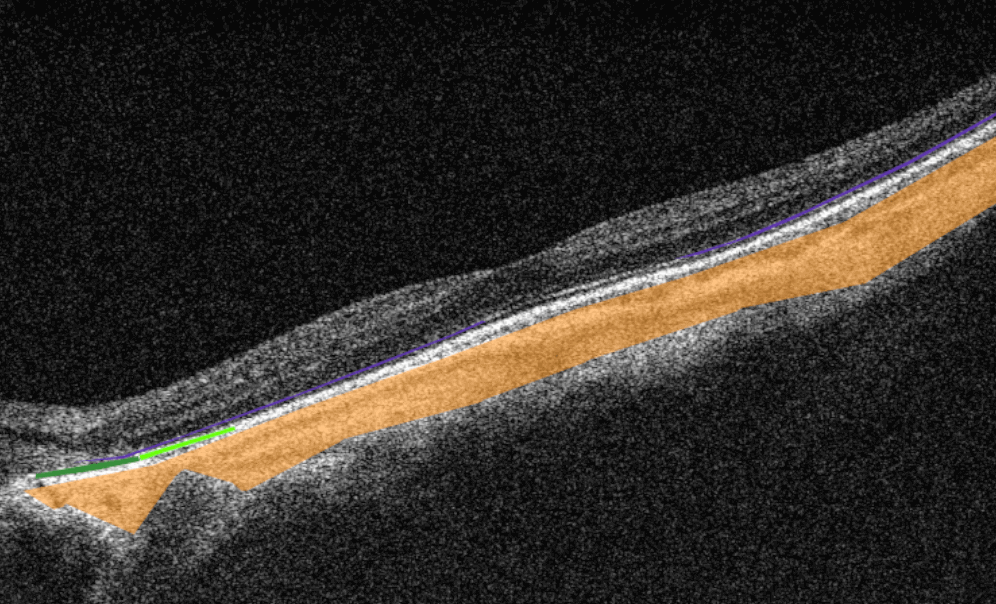
Altris AI is a unique eye clinic management system that allows eye care specialists to analyze OCT scans with the help of artificial intelligence (AI) tools.
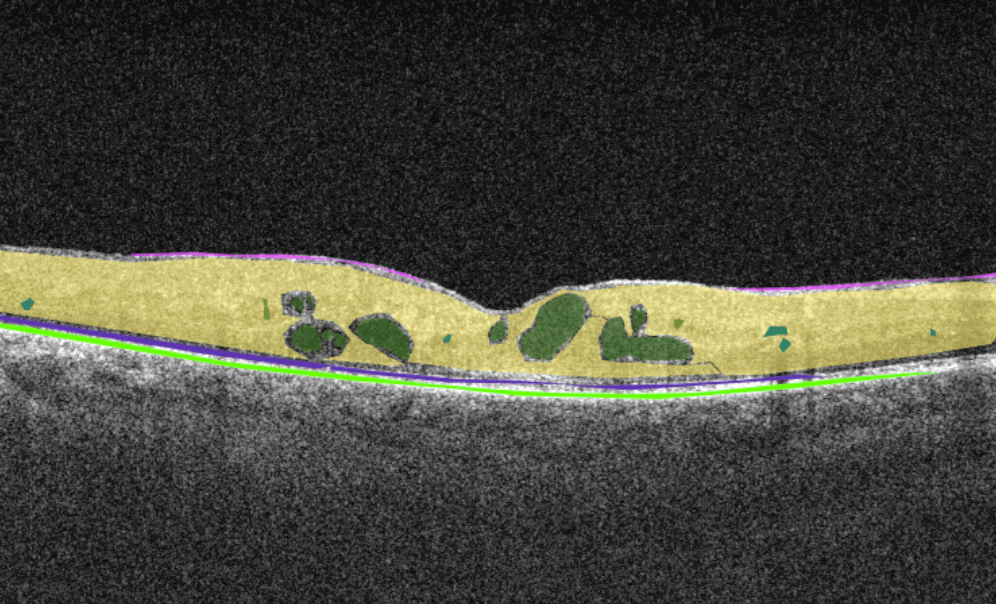
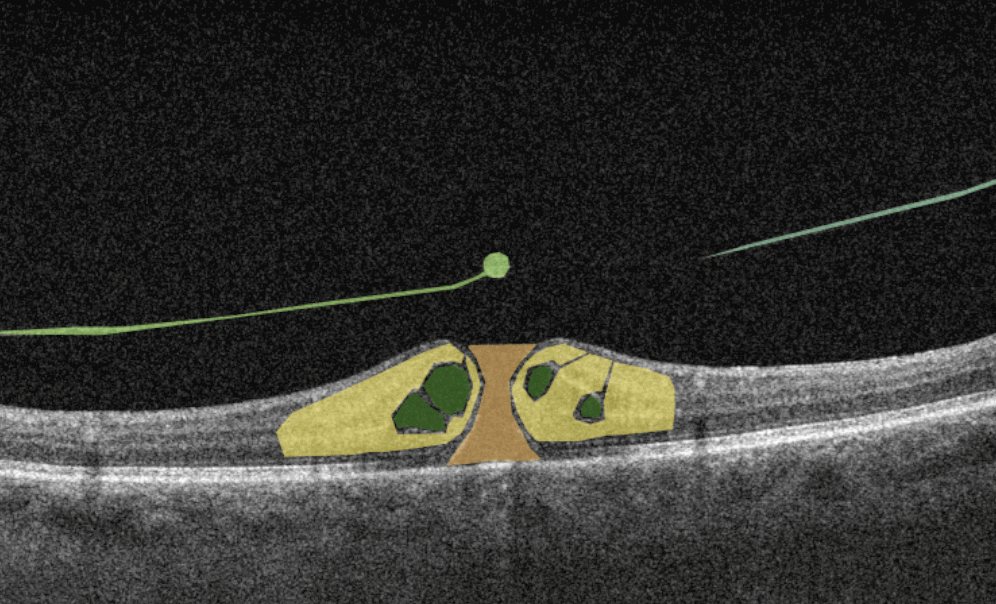
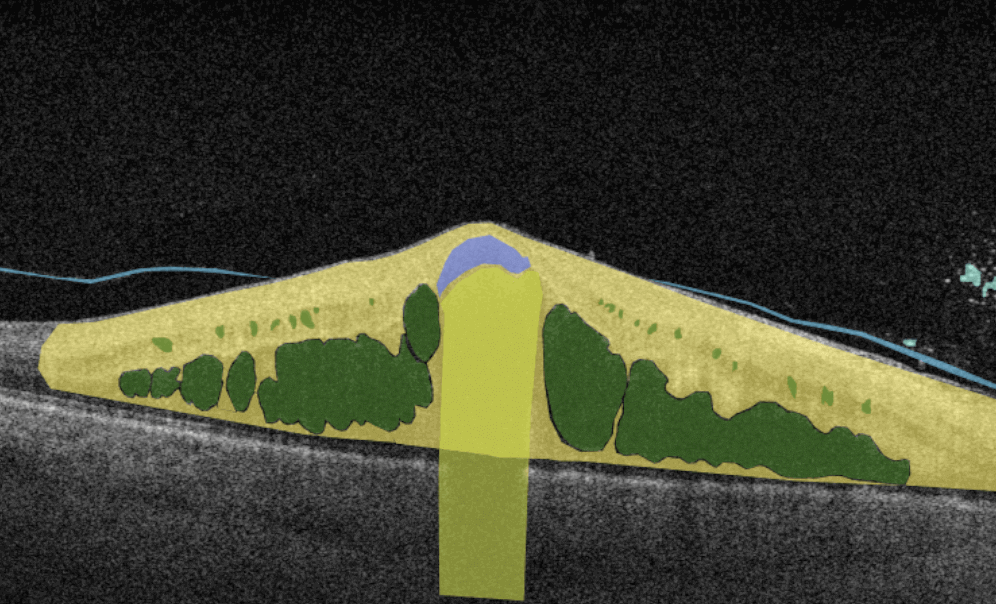
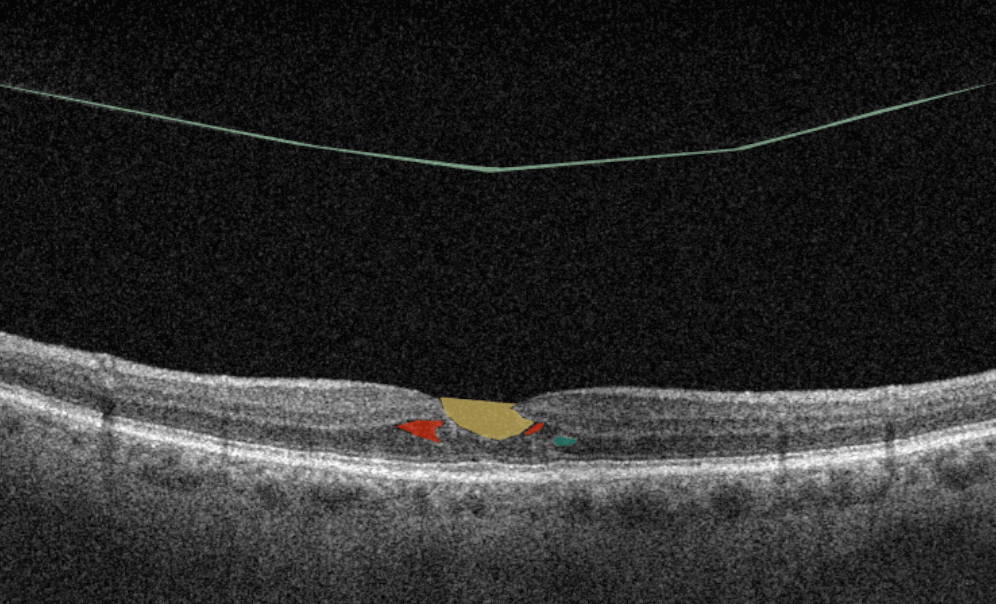
How does it work? Putting it simply, retina specialists have colored thousands of OCT scans and named more than 100 retinal pathologies and pathological signs to train an algorithm, so it can assist specialists in detecting the disease. After loading an OCT scan in the eye hospital management software, the AI model evaluates the b-scans (up to 512) and differentiates between normal scans and scans with moderate and severe pathology. It gives eye care professionals the ability to focus only on serious (red) scans, saving patients valuable time.
In addition, Altris AI allows its users to see a broader perspective of a patient’s eye health. All the reports are dynamically editable: the ophthalmologist can add/revise/delete items in the OCT report. Eye care specialists also can add segmentation/classification results to the OCT report in 1 click. And what’s even more important, Altris AI OCT report is understandable for both ophthalmologists and patients.

AI Decision Support for OCT
Eye clinic management system features of Altris AI
- The system allows working with all popular OCT equipment and all data storage formats, including DICOM of various lengths, png, and jpg.
- Altris AI ophthalmic practice management system can be easily integrated with EHR systems or run standalone as a web application.
- The system also takes care of user security, as all important patient data is tokenized and protected from disclosure at all stages.
- The artificial intelligence program can independently identify more than 100 retinal pathologies and pathological signs.
- The Smart Reports feature allows ophthalmologists to select the elements (single scan, layers, both eyes, etc.) that they want to see in their OCT report.
- This All Scans feature allows the user to view all scans of a single OCT examination, sort them by severity level, and zoom.
Watch a short overview of how Altris AI assists eye care specialists with OCT diagnosis and decision-making.
DrChrono Software

DrChrono EHR is an iPad and iPhone-compatible platform that offers fully customizable form templates or ready-made forms to help users track patient information.
DrChrono EHR is an iPad and iPhone-compatible platform that offers fully customizable form templates or ready-made forms to help users track patient information.
Eye clinic management system features of DrChrono Software
- The system allows medical practices to manage patient admissions, patient care, clinical charts, and billing.
- Healthcare professionals can add patient notes to the medical record. The Vital Flowsheets module provides the ability to create basic health data and monitor the health indicators of each patient.
- The DrChrono eye hospital management software also offers a variety of application integrations.
- Doctors can use the Free Draw module to annotate charts, OCT scans, or other files.
RXNT Software

RXNT is a comprehensive billing, practice management, and EHR solution. This system improves patient care and simplifies clinical management. Access patient health history and prescriptions at the point of care, schedule patients and providers, and request and review lab or imaging orders with multi-site single sign-on (SSO).
Eye clinic management system features of RXNT Software
- Any RXNT ophthalmic practice management system products (EHR, ERX, PM, Billing, Scheduling) can be combined into a fully integrated “Full Suite” system.
- Ophthalmologists, managers, or staff can add and organize documents in patient charts for clinical care plans and follow-up.
- The system has developed customizable “smart” forms and short keys that improve work processes.
- RXNT can share real-time data with other doctors to better coordinate care and support.
In addition, an ophthalmic clinic can integrate RXNT eye hospital management software with the Altris AI system to create and dynamically edit OCT reports.
Medfiles Software

Medfiles Software is a multi-task cloud-based solution that ensures compliance for ophthalmology clinic employees. The key features of this eye hospital management software are drug screening management, medical record tracking, case management, training tools, reporting, and safety documentation.
Eye clinic management system features of Medfiles Software
- Medfiles tracks patient treatment plans, open cases, treatment plans, medical expenses, and cash reserves and creates conclusions based on all the information.
- The system can be easily integrated with different software so a doctor or staff can see scans of specific OCT examinations.
Medfiles eye clinic management system allows to compare annual summary reports with benchmarks.
IntelleChartPRO Software

Another cloud-based ophthalmic electronic medical record (EMR) solution is IntelleChartPRO. This system is very popular among ophthalmology clinics and centers. IntelleChartPRO helps professionals record and manage a patient’s treatment and medical history more effectively.
Eye clinic management system features of IntelleChartPRO Software
- Physicians or ophthalmology clinic management can customize the EHR themselves to fit their unique workflows.
- IntelleChartPRO eye hospital management software developed adaptive template technology that allows offices to generate templates for each patient.
- In combination with other eye clinic management system tools, the software becomes more relevant and allows more accurate diagnoses of patients and the creation of detailed reports.
MaximEyes Software

MaximEyes is a comprehensive, unified electronic health record (EHR) and practices management solution designed exclusively for ophthalmology practices. It has a modern and intuitive user interface. The system will work on any computer OS. If users do not want to use cloud technologies or the clinic has a weak Internet connection, MaximEyes can be deployed through a local server
Eye clinic management system features of MaximEyes Software
- For each patient, the system allows ophthalmologists to set up an individual template according to different types of visits.
- The eye hospital management software EHR includes a flexible rules engine that will suggest or automatically generate post-diagnosis codes, procedure codes, and output documents.
- The First Insight module also offers an ophthalmic imaging management solution that works with any EHR.
75health Software

One more fully-fledged eye clinic management system is 75health, which is also a cloud-based solution that provides its users with electronic health record tools. 75health system will be most suitable for managing health records and patient information for ophthalmologists working in small and mid-sized clinics.
Eye clinic management system features of 75health Software
- 75health eye hospital management software allows ophthalmic clinic staff to download and save patients’ medical images, such as consent forms, handouts, or scans.
- Doctors can also create a treatment plan for their patients right in the system and scan records for allergies, medications, lab results, and symptom lists.
- 75health solution provides smooth integration of ophthalmic management systems, which helps ophthalmologists in decision-making.
myCare Integrity Software

Another cloud-based eye hospital management software that is worth your attention is myCare Integrity. It was created specifically for eye care specialists and contains a strong set of tools and modules that can cover the needs of any member of the ophthalmic clinic staff: from doctors to managers.
Eye clinic management system features of myCare Integrity Software
- The myCare Integrity system has an IntegriVIEW functionality that allows practitioners to link medical images directly to every screen of EMR.
- There is also an IntegriDRAW module inside the eye clinic software, where templates are included in the application. It allows users to rely on the previously created stamps.
- The IntegriLINK module allows ophthalmologists to link the diagnostic equipment to the system.
- What is more, myCare Integrity eye hospital management software allows you to customize and personalize the dashboard.
Summing up
Eye hospital management software is extremely important for any clinic, whether there are 10 or 500 employees. It can help you improve your workflow by keeping a lot of data in one place. Imagine how easily you can get rid of unnecessary paperwork, forget about administrative costs, and speed up processing. In addition, with an ophthalmic practice management system, you can get 24/7 access to patients’ data.

AI Decision Support for OCT
However, the key benefit of practice management software for opticians is the improvement of diagnosis and treatment. There are already ophthalmic image management systems, like Altris AI, that can not only help to manage patients’ data but also provide a second opinion regarding medical image analysis. Using this knowledge, doctors can have better access to patients’ health problems and reports, ultimately enabling them to provide the best care to their clients.
-

The Application of Machine Learning in Ophthalmology: The View from the Tech Side
 Philip Marchenko
30.11.202215 min read
Philip Marchenko
30.11.202215 min readAccording to the World Health Organization (WHO), artificial intelligence (AI) and machine learning (ML) will improve health outcomes by 2025. There are numerous digital technologies that shape the health of the future, yet AI and machine learning in ophthalmology and medical image analysis look like one of the most promising innovations.
The healthcare industry produces millions of medical images: MRI, CT, OCT, images from the lab, etc. The right diagnosis depends on the accuracy of the analysis by the specialists. Today AI can back up any medical specialist in medical image analysis: providing confidence and much-needed second opinion.

Try Altris AI for free
Check how artificial intelligence assists in OCT interpretation
Altris AI team decided to improve medical image analysis for just one type of medical image: Optical Coherence Tomography scans of the retina. To do it, the Altris AI team collected thousands of OCT scans and graphically labeled them, defining more than 100 pathologies and pathological signs. Watch the video to discover more features of Altris AI platform.
Then all this data was fed into the AI model. Further, I will tell how exactly we train the AI model of Altris AI so that it can detect more than 100 pathologies with 91% accuracy, but first, let’s discuss why it is important for the healthcare industry.
Why are automation and machine learning in ophthalmology important?
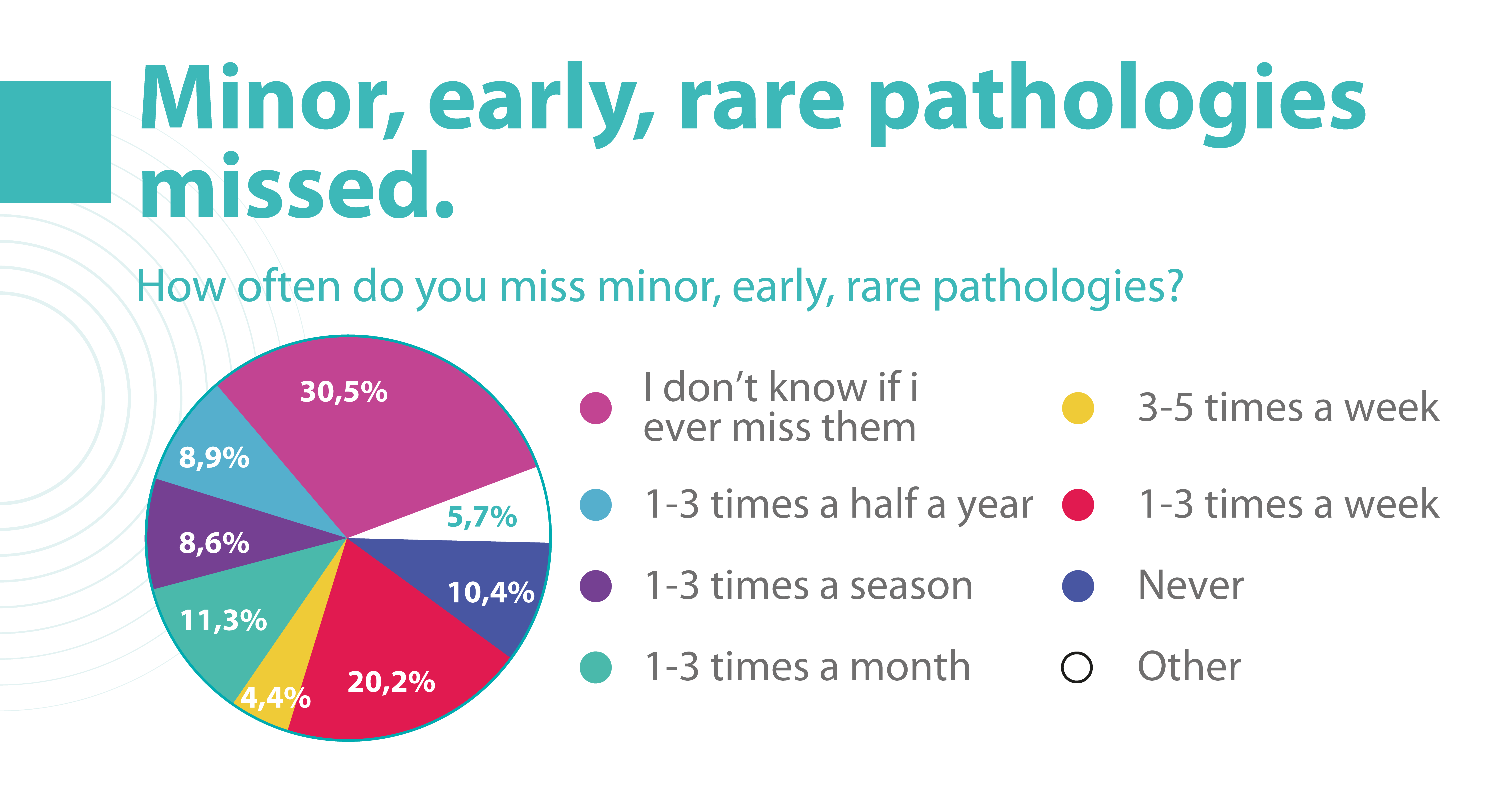
Due to the delicate anatomy of the eye, its treatment carries a high risk of complications. Sometimes these complications can be the result of a medical error by an eye care specialist. But how often?
According to the Altris team research, 20.2% of eye care practitioners miss minor, early, and rare pathologies on OCT scans 1- 3 times a week, and 4.4% miss them 3-5 times a week. But the worst thing is that 30.5% of ophthalmologists and optometrists are not even sure if they are missing any pathology at all.

Some medical errors may be minor, but some may cause significant harm to patients. Such medical errors can lead to medical malpractice lawsuits. That is why most ophthalmic clinics consider implementing AI to double-check the diagnosis of the ophthalmologist.
Besides, different tools of machine learning in ophthalmology have a high level of accuracy and can provide eye care specialists with a second opinion.
How to reach a high level of accuracy?
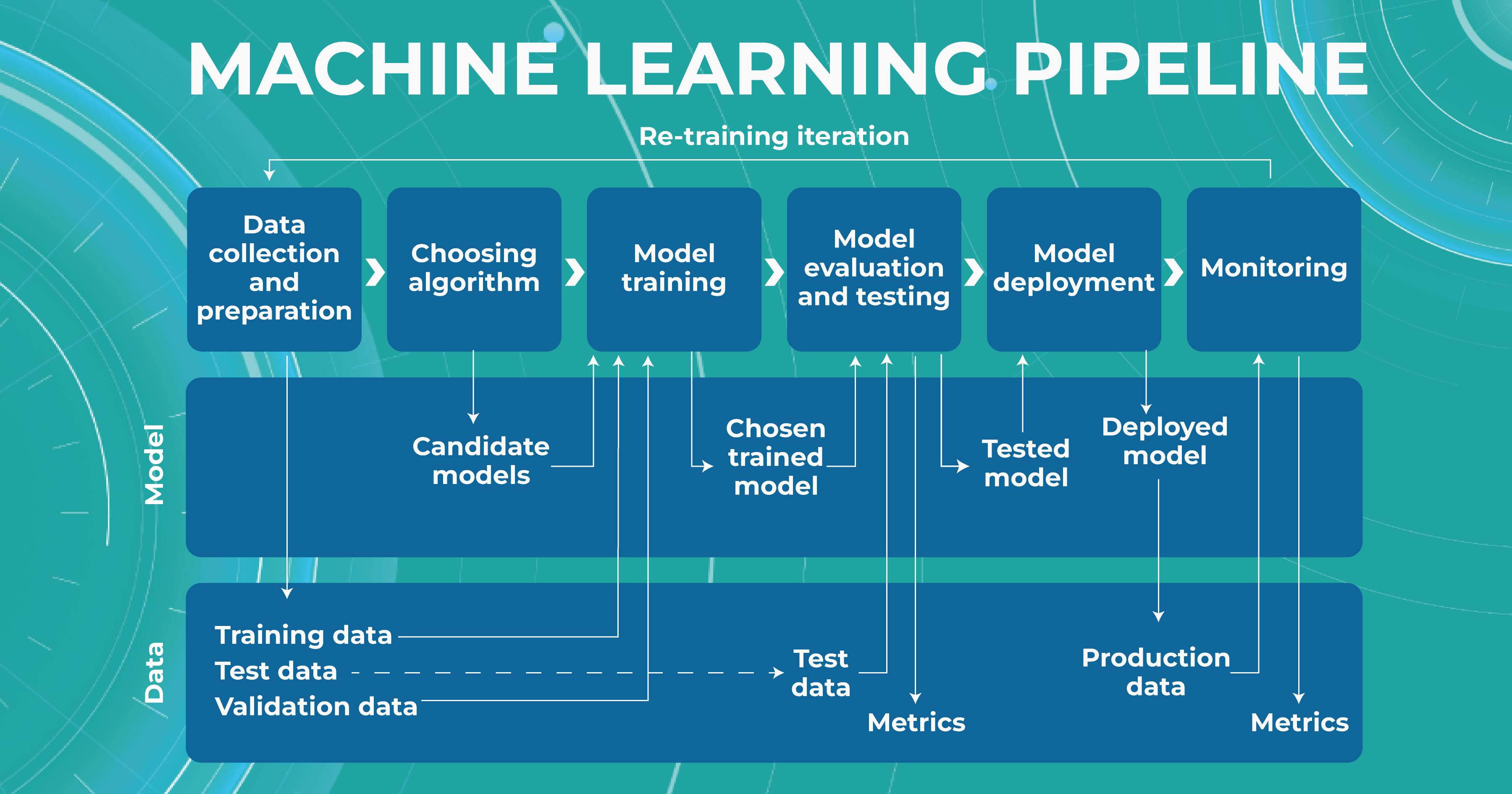
It is almost always necessary to conduct many experiments to achieve a high level of model accuracy (in the case of Altris AI, it is 91%). It is often done with the help of a machine learning pipeline.

High level of ML pipeline accuracy
The machine learning pipeline is programmed by a team of engineers to perform certain steps automatically. It systematically trains and evaluates models, monitors experiments, and works with datasets.
-
- ML and Medical teams collect, annotate and preprocess data. It’s crucial to ensure the data quality is at its highest level because the model’s quality heavily depends on it. To do this, the teams developed a process and annotation guideline, which ensures that the number of errors in the annotation is minimized.
- ML team chooses the appropriate approach (model) depending on the collected data and the tasks. Each team member is well-versed in the most modern and high-quality approaches that solve emerging tasks.
- The selected model is trained on the annotated data.
- In the model evaluating and testing stage, we develop tests aimed at helping us understand whether the model is trained properly to perform the needed tasks.
- After the ML team is satisfied with the result, we deploy the model, which means the model is ready for production.
- While the model is running in production, we monitor its performance to ensure everything goes well.
This workflow allows engineers to continuously fine-tune existing models alongside constant performance evaluations. The most significant advantage of this process is that it can be automated with the help of available tools.

Try Altris AI for free
Check how artificial intelligence assists in OCT interpretation
What tasks does machine learning in ophthalmology have?
Within the Altris AI platform, we solve 2 main tasks: segmentation and classification of OCT scans.
Classification task
Classification is the task of determining which category a particular object belongs to. We assign each pathology to a certain class of pathologies (for example, glaucoma class).
Segmentation task
The image segmentation problem can be stated as the division of an image into regions that separate different objects from each other, and from the background.
Key metrics of Altris ML pipeline
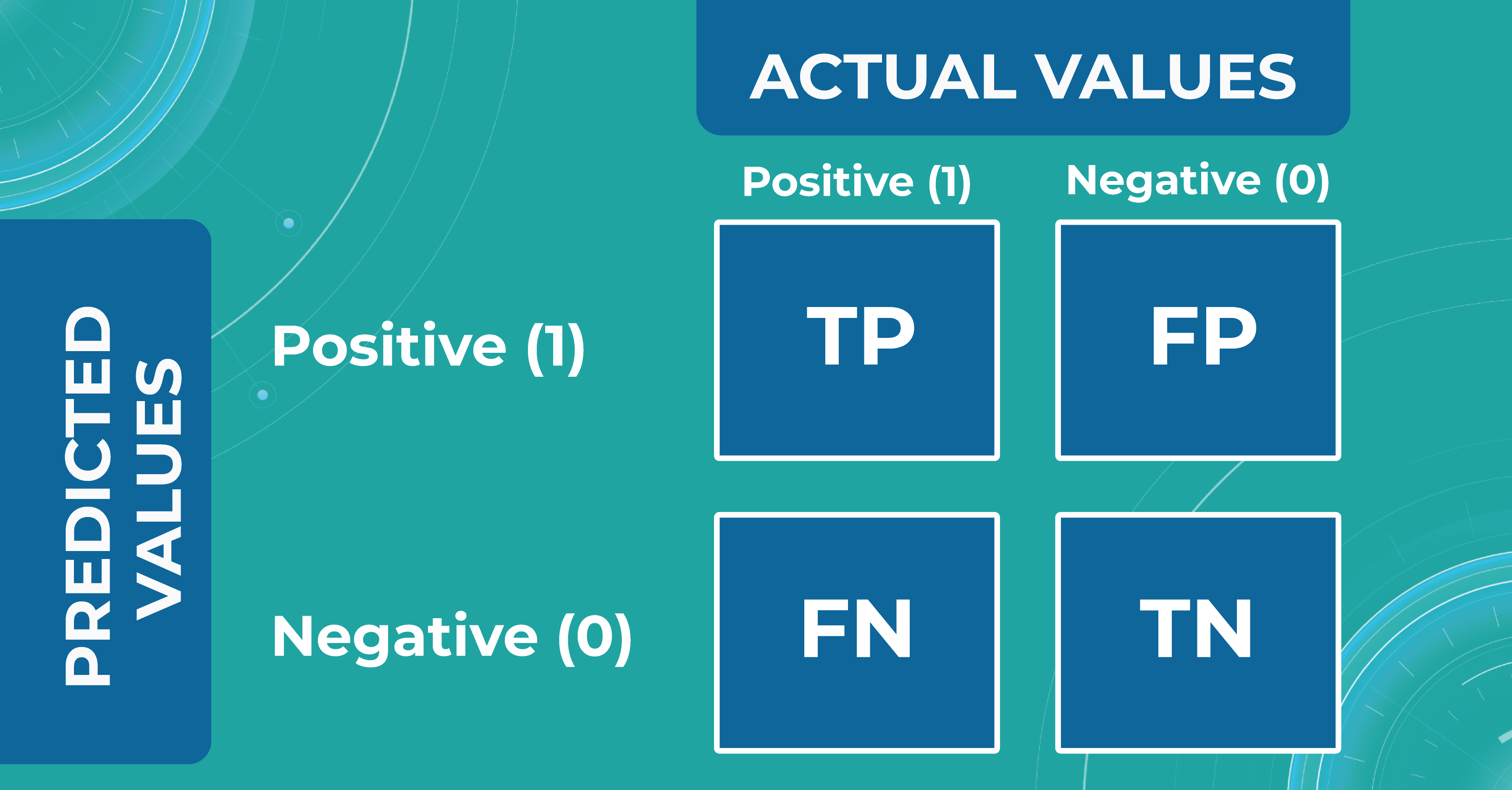
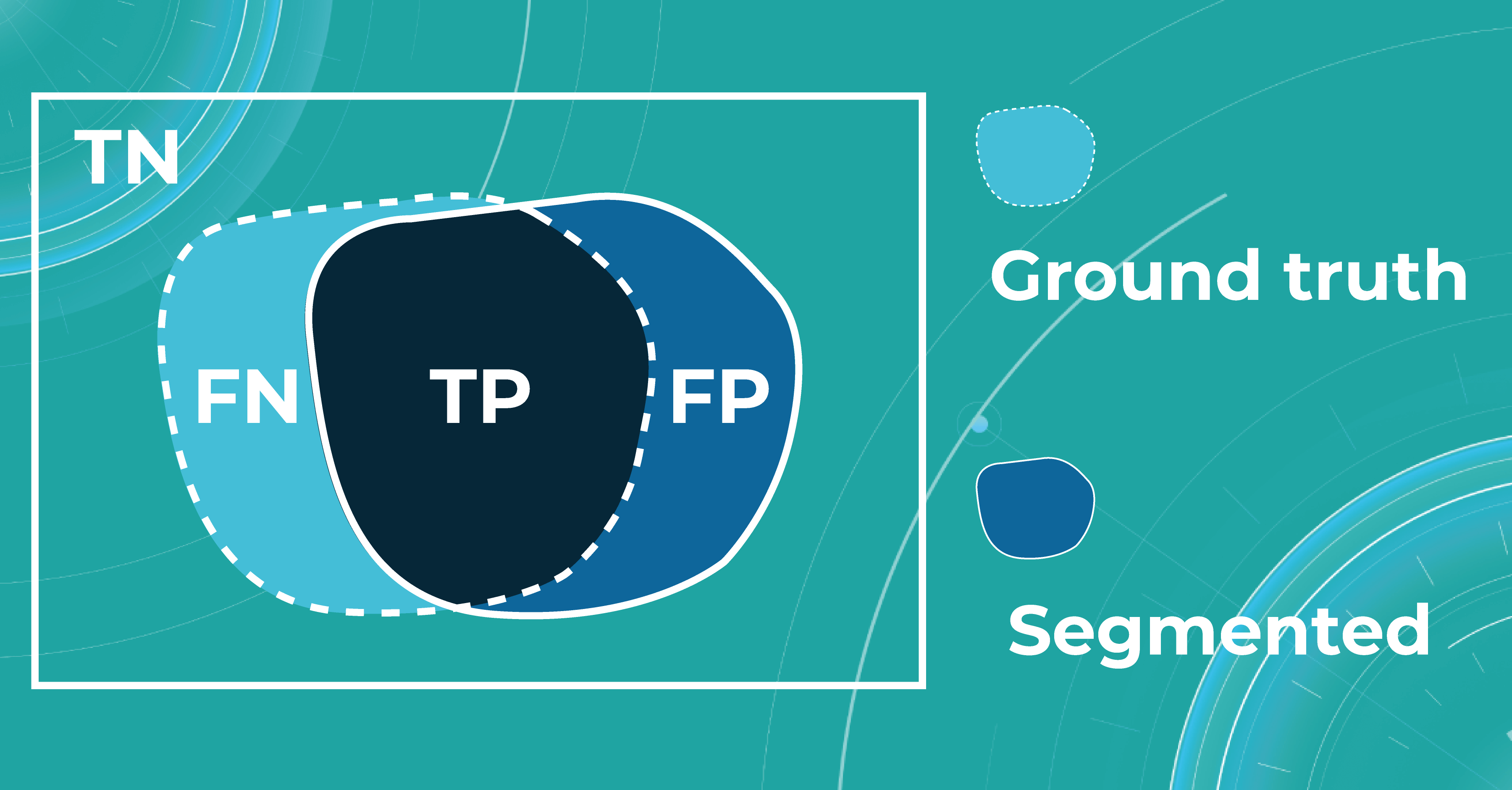
When discussing classification and segmentation metrics in medical imaging machine learning, it is essential to mention the Confusion matrix (CM). CM is a visualization of our performance, which helps us understand whether the model is performing well in terms of predicted and real data. For a better explanation, let’s take a look at the picture.

Let’s consider 4 possible outcomes from model predictions. Say we need to create a classifier to diagnose or predict if a patient has a disease (positive / 1 or TRUE) or not (negative/ 0 or FALSE). In such a case, the model can predict “yes” or “no”, and we can have an actual “yes” or “no”. Based on this, we can get 4 categories of results:
- TP — true positive. The patient that actually has a disease has been diagnosed with this disease. A class was predicted to be true, and it is actually true.
- TN — true negative. The patient is actually healthy and has been diagnosed as healthy. A class was predicted to be false, and it is actually false.
- FP — false positive (type 1 error). The patient that is actually healthy has been diagnosed as having a disease. A class was predicted to be true, but it is actually false.
- FN — false negative (type 2 error). The patient that actually has a disease has been diagnosed as healthy. A class was predicted to be false, but it is actually true.
With the help of the confusion matrix, our ML engineers get specific metrics needed to train our medical imaging machine learning model properly. We discuss each metric in more detail in the following paragraphs.
Classification metrics
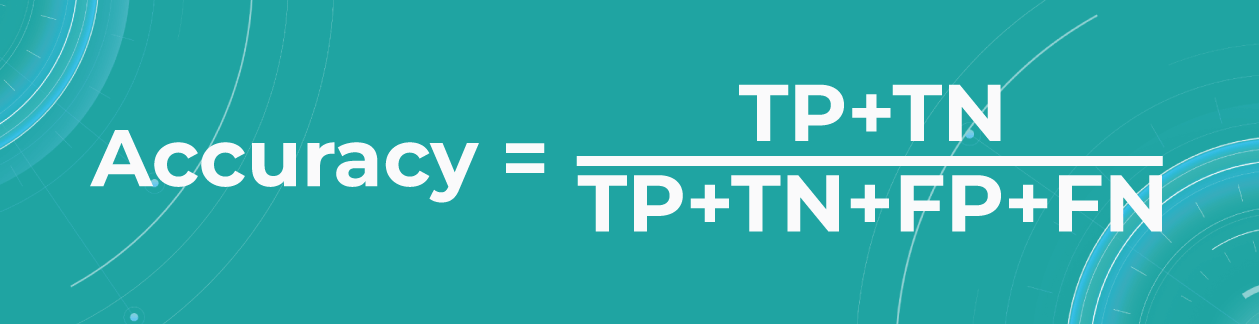
- Accuracy
To find out how many of our predictions were correct, we divide the number of correct predictions by the total.

While being intuitive, the accuracy metric heavily relies on data specifics. If the dataset is imbalanced (the classes in a dataset are presented unevenly), we won’t get trustful results.
For example, if we have a training dataset with 98% samples of class A (healthy patients) and only 2% samples of class B (sick patients). The medical imaging machine learning model can easily give you 98% training accuracy by predicting that every patient is healthy, even if they have a disease. Such results may have destructive consequences as people won’t get needed medical treatment.
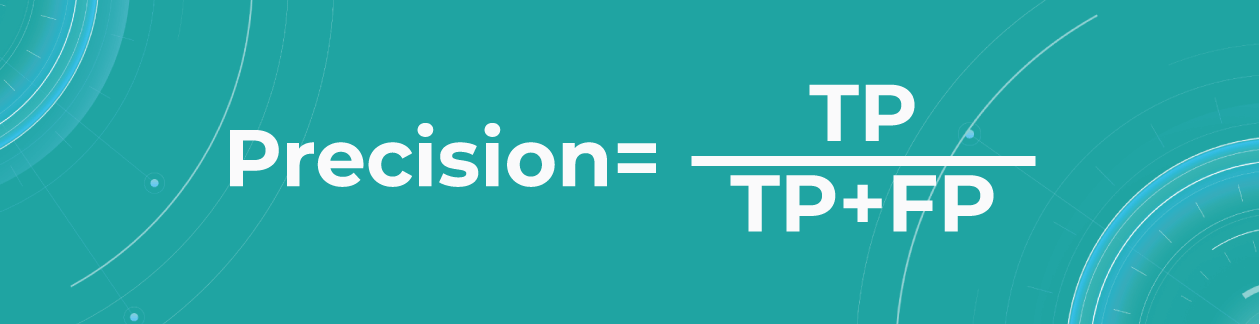
- Precision
Precision shows what proportion out of all positive predictions was correct.

Precision metric helps us in cases when we need to avoid False Negatives but can’t ignore False Positives. A typical example of this is a spam detector model. As engineers, we would be satisfied if the model sent a couple of spam letters to the inbox. However, sending an important non-spam letter to the spam folder (False Positive) is much worse.
- Sensitivity/Recall
Recall shows how many of all really sick patients we predicted and diagnosed correctly. It is a proportion of correctly positive predictions out of all positives.

In our case, you want to find all sick people, so it would not be so critical if the model diagnoses some healthy people as unhealthy. They would probably be sent to take some extra tests, which is annoying but not critical. But it’s much worse if the model diagnoses sick people as healthy and leaves them without treatment.
The sensitivity of Altris AI is 92,51%
- Specificity
The specificity shows how many of all healthy patients we predicted correctly. It is the proportion of actual negatives that the medical imaging machine learning model has correctly identified as such out of all negatives.

Specificity should be the metric of choice if you must cover all true negatives and you can’t tolerate any false positives as a result. For example, we’re making a fraud detector model in which all people whose credit card has been flagged as fraudulent (positive) will immediately go to jail. You don’t want to put innocent people behind bars, meaning false positives here are unacceptable.
The specificity of Altris AI is 99,80%
Segmentation metrics
Segmentation also can be thought of as a classification task. For each pixel, we make predictions about whether it is a certain object or not. Therefore, we can talk about Accuracy, Precision, Recall, and Specificity in terms of segmentation.
Let’s say we have a Ground Truth (what is really an object) and a Segmented (what the model predicted). The intersection in the picture below is the correct operation of the medical imaging machine learning model. All that is the difference (FN and FP) is the incorrect operation of the model. True negative (TN) is everything the model did not mark in this case.

Quite often, even after looking at such metrics, the problem of non-symmetricity remains in the segmentation tasks. For example, if we consider a tiny object, the Accuracy metric doesn’t work. Therefore, segmentation tasks also refer to additional metrics that allow taking into account the size of the object of the overall quality assessment. Let’s look at additional metrics in more detail.
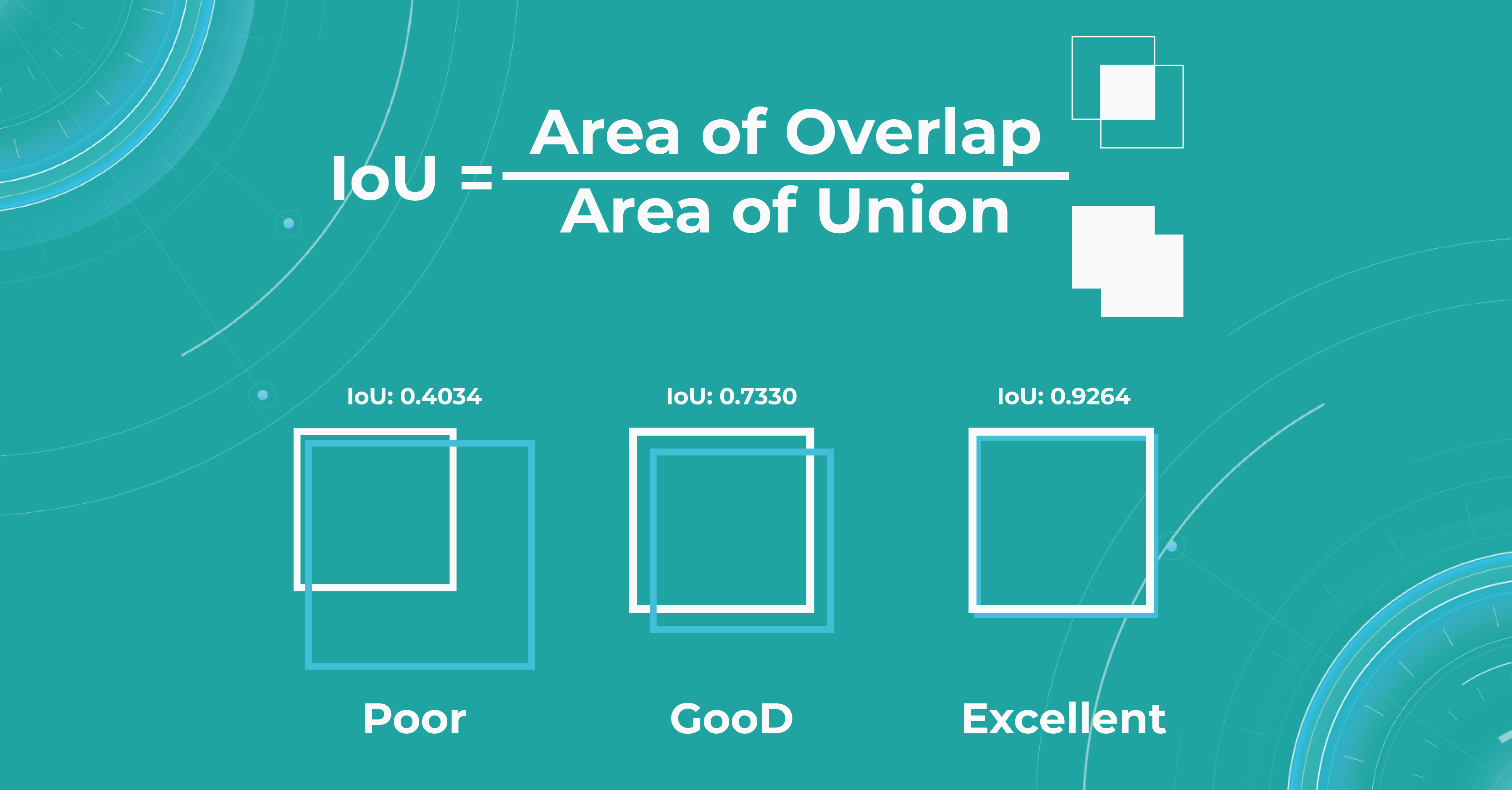
- Intersection over Union (IoU)/Jaccard
Intersection over Union is an evaluation metric used to measure segmentation accuracy on a particular image. This metric is considered quite simple — the intersection zone is divided by the union of Ground Truth and Segmented.

Sometimes we get such results, like if the object was determined to be very large, but in fact, we see that it is small. Then the metric will be low, and vice versa. If the masks are approximately equal to each other, everything works correctly, and the metric will be high.
- Dice score/F1
The dice coefficient is 2 times the area of overlap divided by the total number of pixels in both images.

This metric is a slight modification of the previous one. The difference is that, in this case, we take the intersection area twice.
Calculating scores over dataset
We calculate the metrics described above for each scan. In order to count them over the entire dataset, we take each picture in this dataset, segment it, calculate the metric, and then take the average value of the metrics on each image.
What is model validation in ML?
In addition to evaluating the metrics, we also need to design the model validation procedure suitable for a specific task.
When we have determined the metric that suits the task of machine learning for medical image analysis, we also need to understand what data to use for calculation. It will be wrong to calculate the metric on the training data because the model has already seen it. This means that we will not check the ability of the model to generalize in any way. Thus, we need a specific test dataset so that we can carry out quality control according to the selected metrics.
The main tasks of the model validation are:
- To provide an unbiased estimation of the accuracy that the model can achieve
- To check whether the model is not overfitted
Picking the correct model validation process is critical to guarantee the exactness of the validation method. In addition, there is no single suitable validation method for machine learning in ophthalmology — each task requires different validation. Engineers separately examine each task to see if data has leaked from the train dataset to the test dataset because this may lead to an overly optimistic estimate of the metrics.
For example, we can take OCT images in different resolutions. We may need a higher image resolution for some diseases. If the medical imaging machine learning model overfits at the resolution of this OCT, it will be called a leak because the model should behave the same at any resolution.
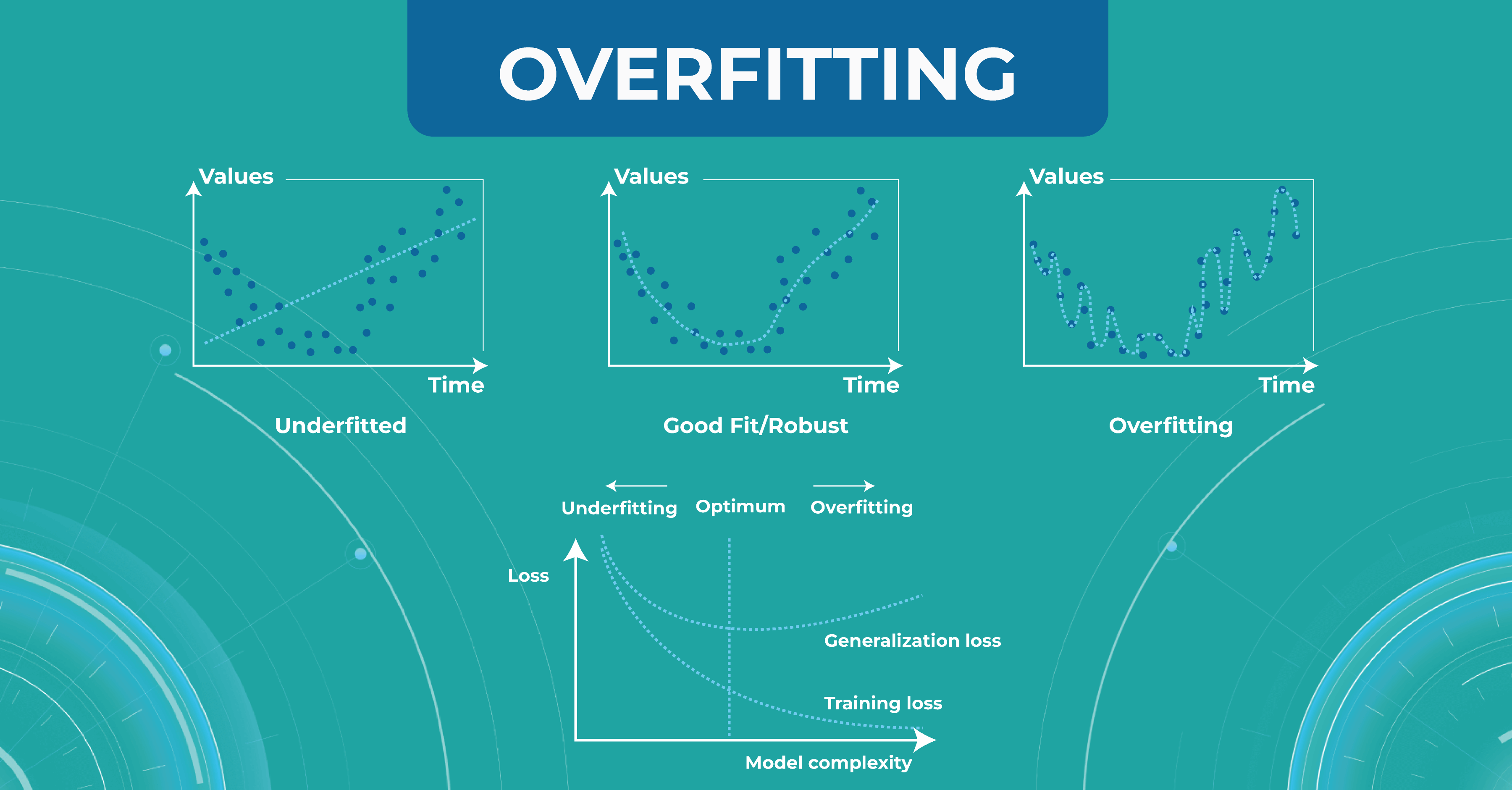
Overfitting and underfitting
The model also has such an important property as a generalization. If the model did not see some data during training, it should not be difficult for the model to determine which class a certain image belongs to.
At this stage, engineers may have two problems that they need to solve. The first problem is overfitting. When the model remembers the training data too well, we lose the ability to make correct predictions. The picture below illustrates this problem. The chart in the middle is a good fit when the model is general enough and has a positive trend, and the trend is well-learned. But the chart on the right shows a too-specific model that will not be able to guess the trend.

Another problem to solve is underfitting. This problem arises when we have chosen a model that is not complex enough to describe the trend in the data (left chart).
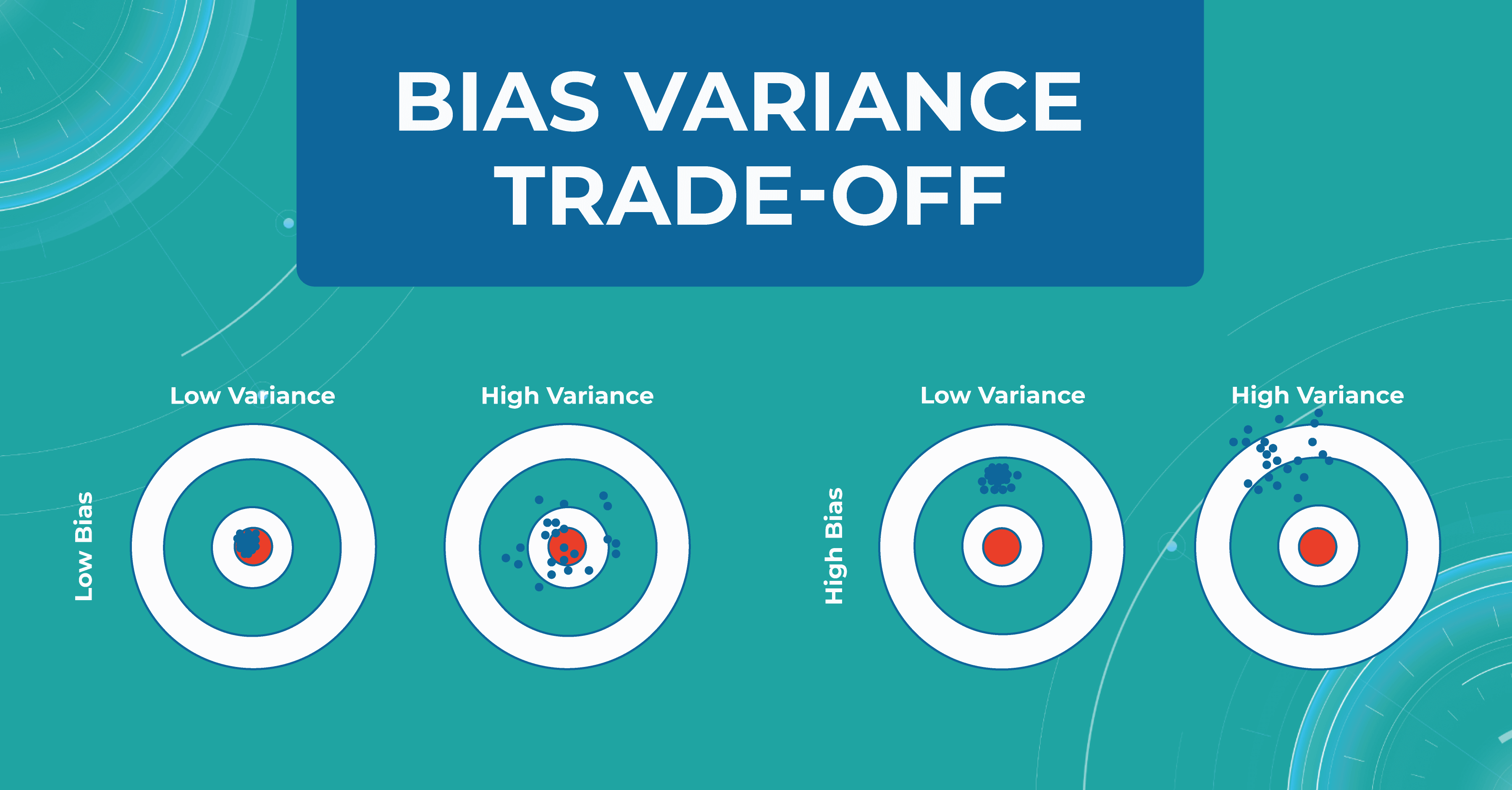
Bias variance trade-off
Another important concept we use in machine learning model validation is the bias variance trade-off. We want our models to always make accurate predictions and have no ground truth scattered. As shown at first/second circle.
However, there are situations when we have a model that predicts something close to the target, but from dataset to dataset, it has a strong scatter. This is showcased in the second circle.
In circle three, you can observe a situation where the model has heap predictions on different datasets, but they are inaccurate. This situation usually indicates that we need to almost entirely rebuild the model.

Overfitting and bias variance trade-off are very important in working with the model, as they allow us to track errors and select a model that will balance between spread and hitting the target.
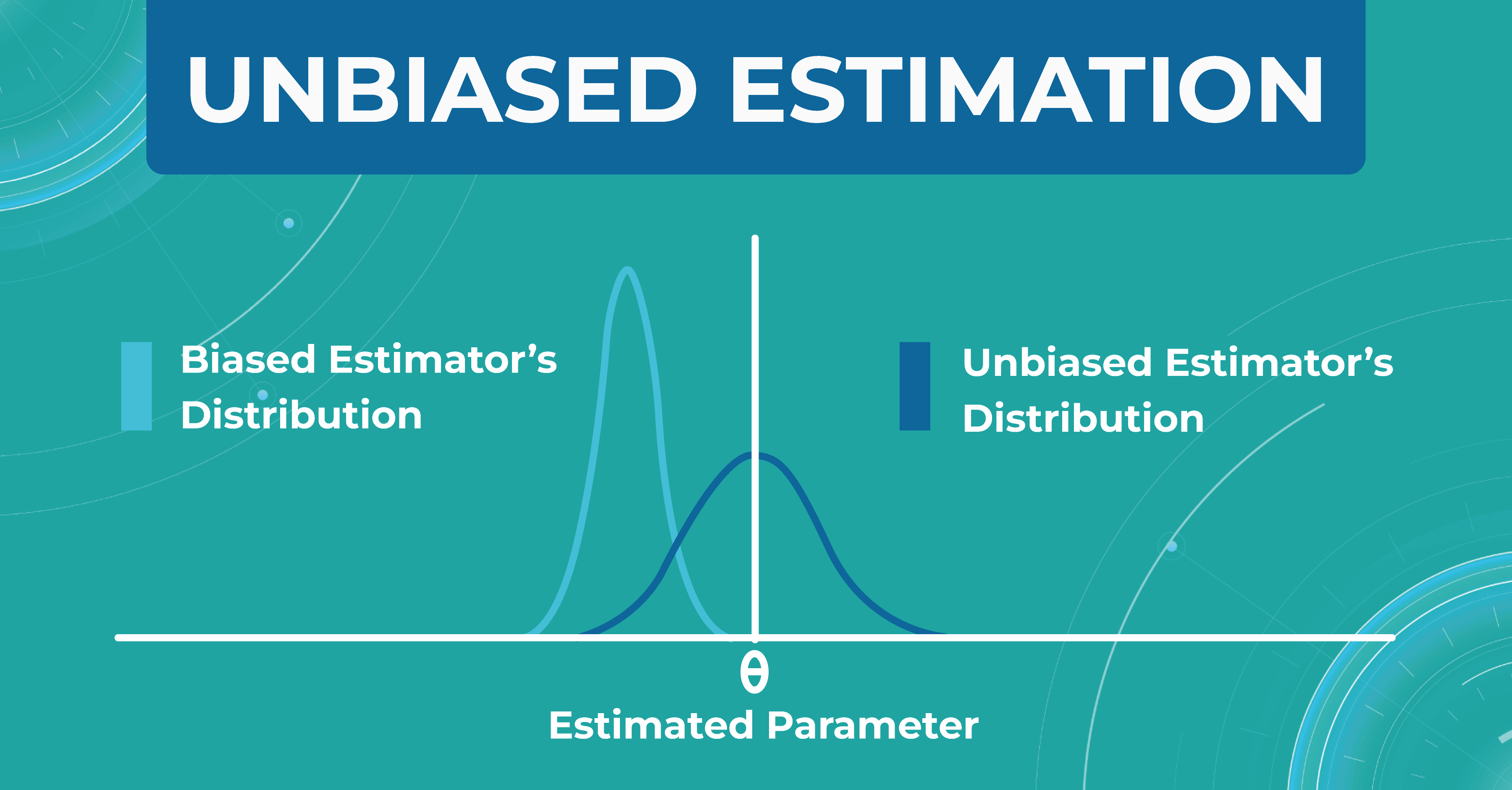
Unbiased estimation
In addition, within each model, we evaluate a set of parameters. We made a certain estimate (graph on the left), but in real life, the distribution of parameters differs (graph on the right). Thus, seeing that our estimate turned out to be shifted, we find another problem that needs to be solved. Machine learning in ophthalmology needs to make the estimate as unbiased as possible.

How do we validate the Altris AI model?
There are three main steps in choosing a validation strategy for machine learning in ophthalmology:
- we got familiar with ophthalmology, understood the nature of data, and where the leakages are possible;
- We estimated the dataset size and target distribution;
- understood the model’s training complexity (amount of operations/ number of parameters/ time) to pick the validation algorithms.
After that, we have a reliable strategy for the machine learning model validation. Here are some fundamental concepts we use in the validation of models’ performance.
Train/test split
Train/test split is the most simple and basic strategy that we use to evaluate the model quality. This strategy splits the data into train and test and is used on small datasets. For example, we have a dataset of 1000 pictures, 700 of which we leave for training and 300 we take for the test.
This method is good enough for prototyping. However, we don’t have enough datasets with it to do a simple double-check. This phenomenon is called high sampling bias: this happens when we encounter some kind of systematic error that did not fit into the distribution in the train or test.
By dividing data into train and test, we are trying to simulate how the model works in the real world. But if we randomly split the data into train and test, our test sample will be far from the real one. This can be corrected by constructing several test samples from the number of data we have and examining the model performance.
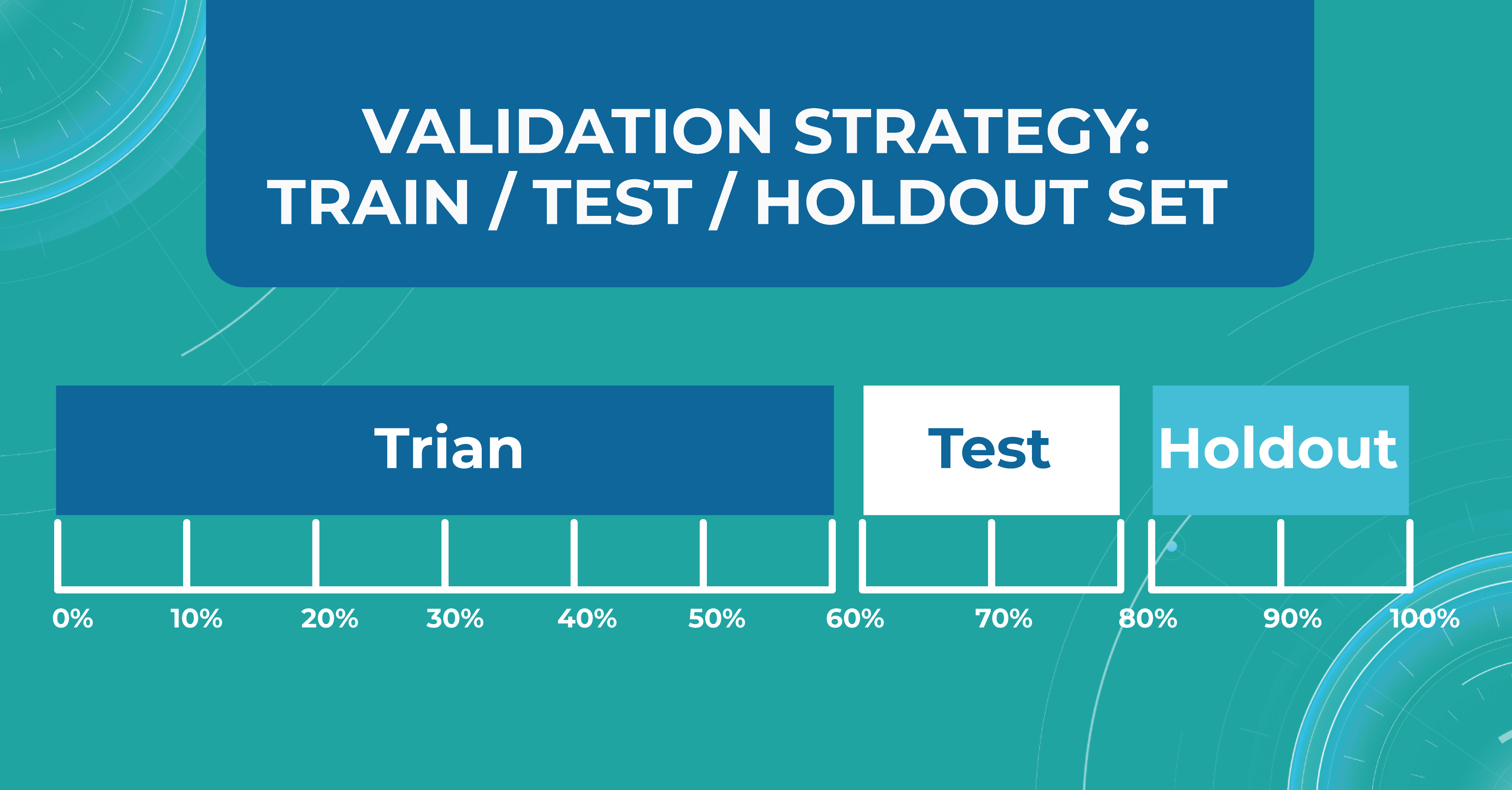
Train/test/holdout set
We leave the holdout as the final validation and use the train and test to work with the medical imaging machine learning model. After optimizing our model on the train/test split, we can check if we didn’t overfit it by validating our holdout set.

Using a holdout as a final sample helps us look at multiple test data distributions and see how much the models will differ.
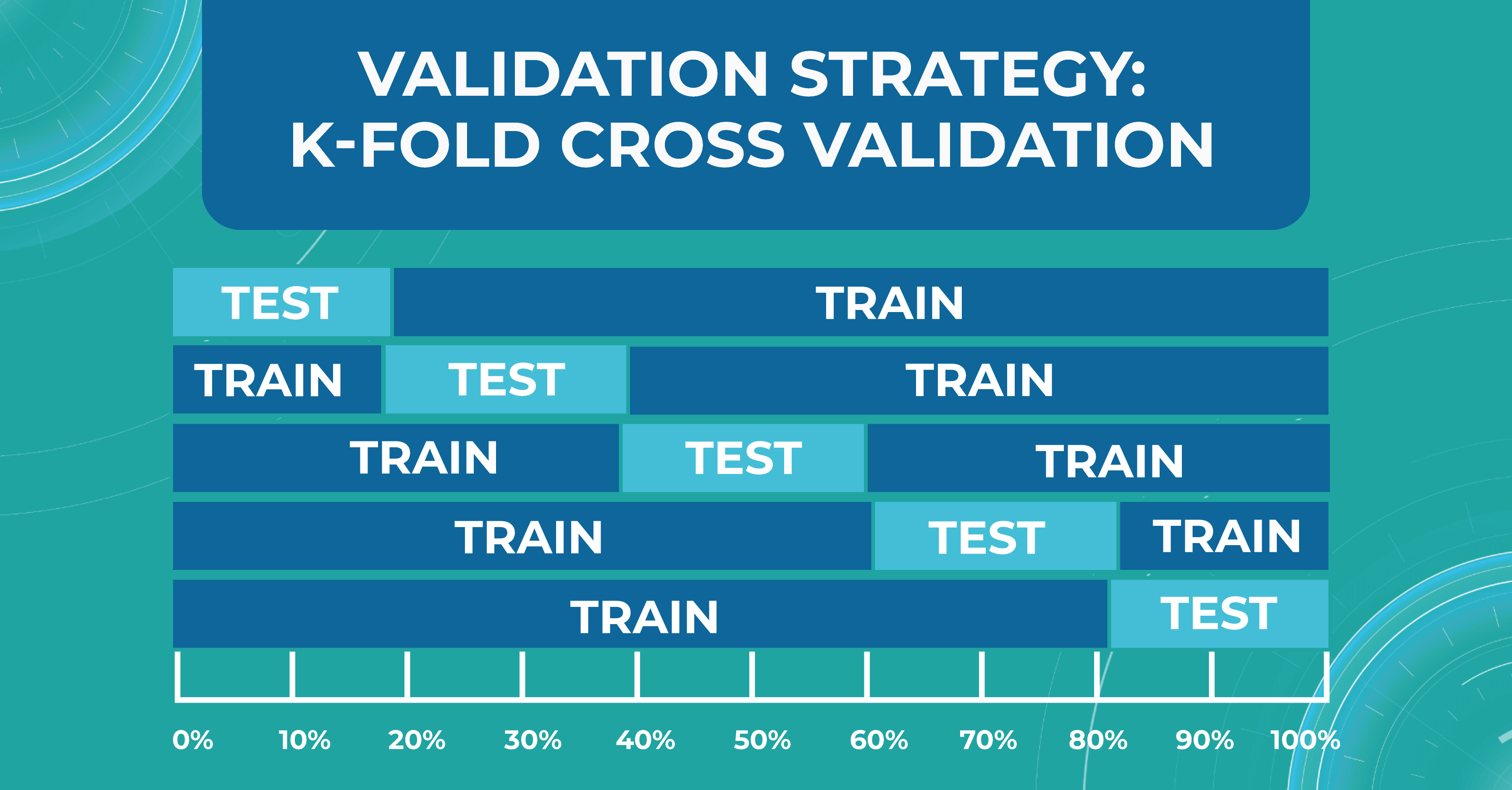
K-fold cross validation
There is also a more general approach that Altris AI team use for validation — k-fold cross validation. This method divides all of our data equally into train and test.

We take the first part of the data and declare it as a test, then the second, and so on. Thus, we can train the model on each such division and see how it performs. We look at the variance and standard deviation of the resulting folds as it will give information about the stability of the model across different data inputs.
Do we need ML models to perform on par with doctors?
Here I will try to answer a question that worries many ophthalmologists and optometrists: can machine learning for medical image analysis surpasses an eye care specialist in assessing quality?
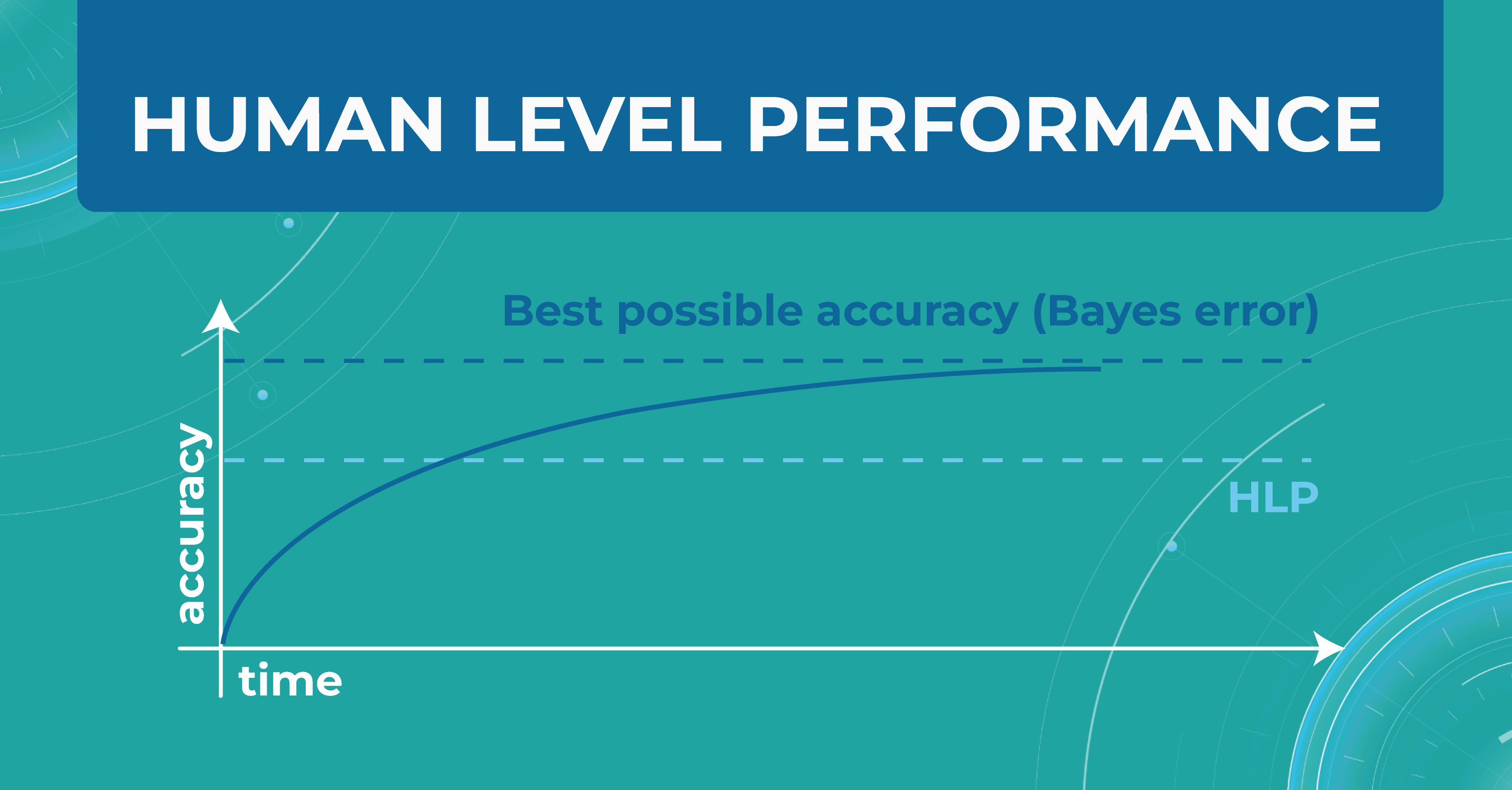
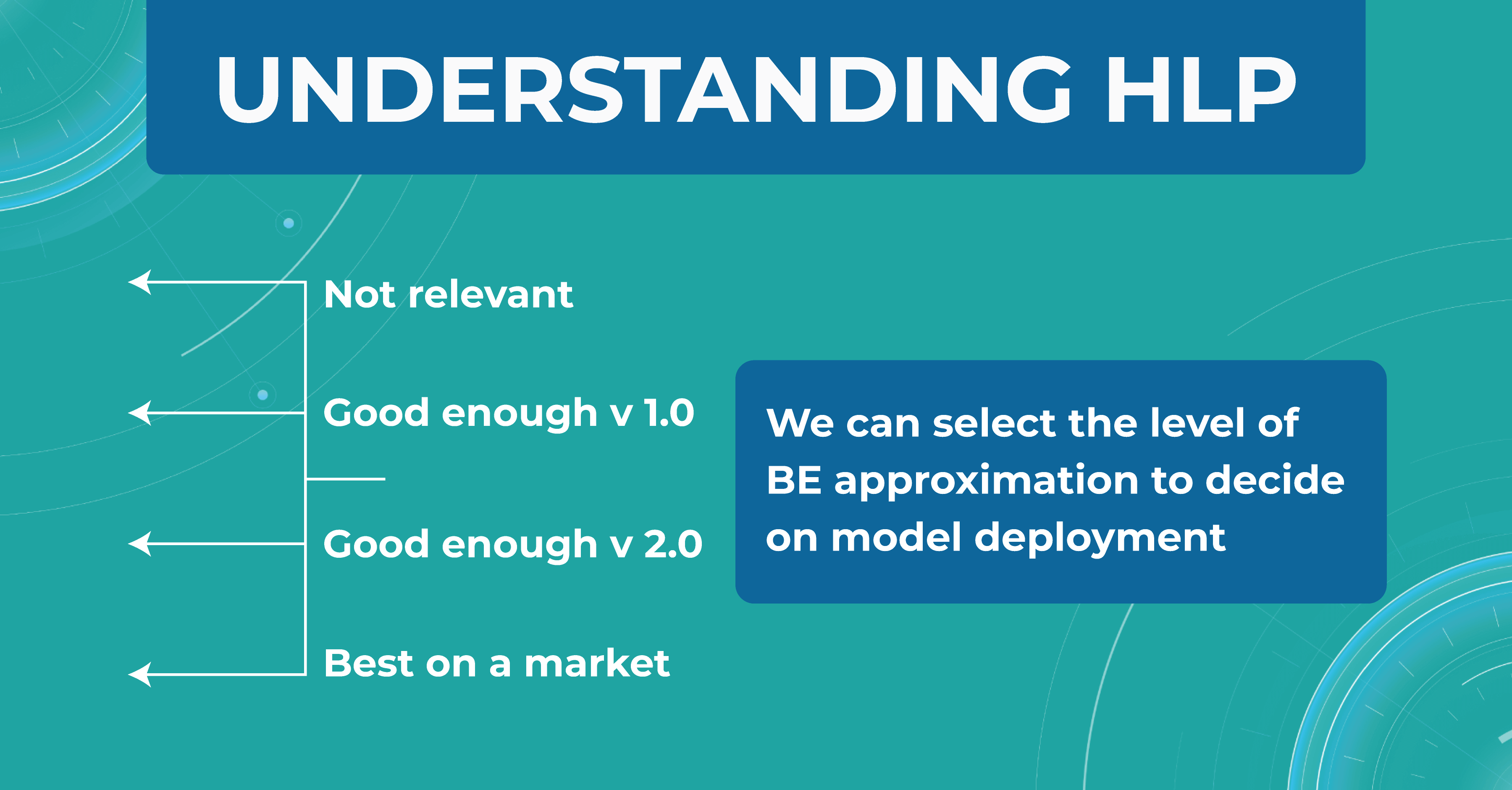
In the diagram below, I have drawn an asymptote called the Best possible accuracy that can be achieved in solving a particular problem. We also have a Human level performance (HLP), which represents how a person can solve this problem.
HLP is the benchmark that the ML model strives for. Unlike the Best possible accuracy, for which there is no formula, HLP can be easily calculated. Therefore, we assume that if a model crosses the human quality level, we have already achieved the best possible quality for that model. Accordingly, we can try to approximate the Best possible accuracy with the HLP metric. And depending on this, we understand whether our model performs better or worse.

For those tasks that people do better and the ML model does worse, we do the following:
- collect more data
- run manual error analysis
- do better bias/variance analysis
But when the model crosses the HLP quality level, it is not entirely clear what to do next with the model and how to evaluate it further. So, in reality, we don’t need the model to outperform a human in interpreting images. We simply won’t know how to judge the quality of this model and whether it can be 100% objective and unbiased.
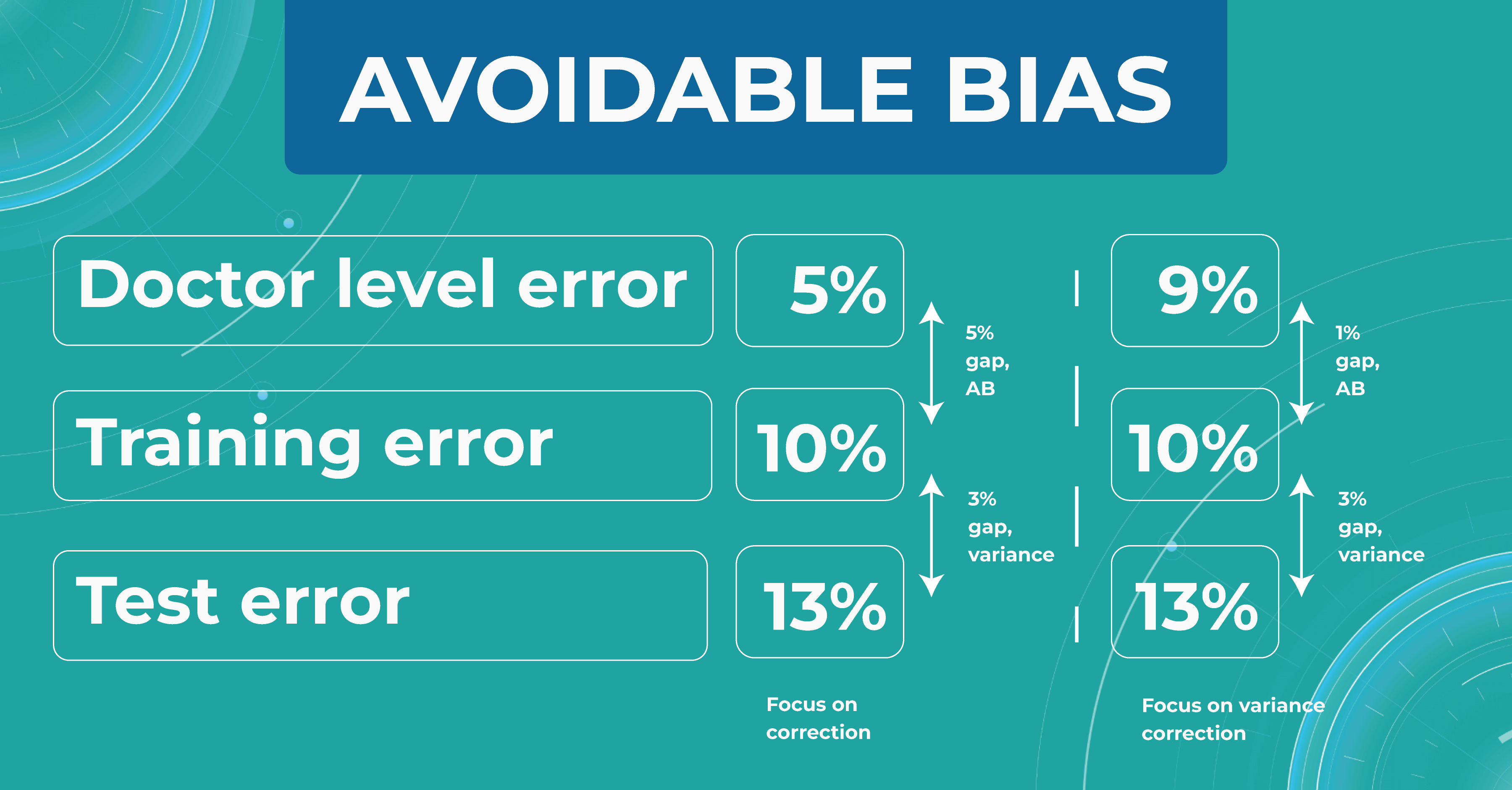
Avoidable bias
Let’s say we need to build a classifier for diabetic retinopathy based on OCT scans, and we have a control dataset prepared by people. In the first case, doctors are wrong 5% of the time. At the same time, the model on the train set is wrong in 10% of cases and on the test set — in 13%.

The difference between the model’s and the human’s performance is usually taken as the minimum difference between the train/test set and the human. In our case, it is 5% gap (10% – 5%) of avoidable bias. It is called avoidable bias because it can be fixed theoretically. In such a case, we need to take a more complex model and more data to better train the model.
In the second case, doctors determine the disease with a 9% error. If the model defines a disease with the same rates as the previous example, then the difference between the train/test set and the human will be 1% (10% – 9%), which is much better than avoidable bias.
Looking at these two cases, we must choose a strategy that will lower the variance for the machine learning model so that it works stably on different test sets. Thus, taking into account the avoidable bias and the variance between the samples, we can build a strategy for training the model so that it could potentially outperform the HLP someday. However, do we need it now?
Understanding HLP
To better understand the HLP metric, let’s consider the task of determining dry AMD on OCT scans. We have a fixed dataset and 4 train sets, each one determining dry AMD with a specific accuracy:
- ML engineers – 20%
- ophthalmologists – 5%
- 2 ophthalmologists – 3%
- 2 ophthalmologists and 1 professor of ophthalmology – 2%

We take a result of 2% as the best HLP possible. To develop our model, we can choose the performances we strive to get. The 20% error result is irrelevant, so we discard this option. But the level of 1 doctor is enough for model version number 1 model. Thus, we are building a development strategy for model 1.
Summing up
Machine learning will revolutionize the eye care industry. It provides confidence and second opinion to eye care specialists in medical image analysis.
If you are looking for ways to use machine learning in your eye care practice, feel free to contact us. At Altris AI, we improve the diagnostic process for eye care practitioners by automating the detection of 54 pathological signs and 49 pathologies on OCT images.
-
-

Altris AI Builds Partnership with Academic Institutions
 Maria Znamenska
21.11.20222 min read
Maria Znamenska
21.11.20222 min readAI in ophthalmology for academic purposes
Aston University and Altris AI join forces to Revolutionise Optometry Education
We are proud to announce our new strategic partnership with Aston University, a renowned healthcare education and research institution. This partnership marks a significant step forward in enhancing the preparation and training of optometry students with the help of AI technology.
As OCT examination proves to be one of the most accurate and yet the most complex diagnostic devices in the eye care industry, it is crucial for educational institutions to stay ahead and equip their students with the most innovative tools, such as artificial intelligence. The collaboration between ourselves and Aston University will enhance how optometry students learn and improve OCT interpretation skills.
Aston University, known for its commitment to excellence in healthcare education, has chosen to partner with Altris AI to integrate AI-driven solutions into future optometrists training in the lecture theatre, university clinics, and research departments. The Altris AI platform will also be used in the study of Ph.D.-level research projects.
Commenting on this exciting partnership, James Wolffsohn, Head School of Optometry said “We strive to equip our students with cutting edge knowledge and tools to deliver world class eyecare to their patients. This partnership with Altris AI will help strengthen our students diagnostic ability and keep on the crest of the innovation wave offered by AI”
Maria Znamenska, MD, Ph.D., Associate Professor of Ophthalmology and a Chief Medical Officer at Altris AI, expressed her enthusiasm, stating, “The new generation of optometry students ask for modern ways of learning. Today they want more than books and atlases, they want to learn interactively and utilise the power of technology in clinical practice. And we are happy that AI has become a true copilot for the young generation of optometry students at Aston University.”
This partnership is a testament to the commitment of both organisations to innovation in healthcare education. Together, Aston University and Altris AI aim to shape the future of optometry education and empower students to provide excellent level eye care services to patients. After all, the ultimate goal of digitalisation in healthcare is always healthier patients.
Contact us
Ask us any question -

Will Artificial Intelligence Replace Ophthalmologists & Optometrists?
 Maria Znamenska
17.11.20228 min read
Maria Znamenska
17.11.20228 min readWill optometrists be replaced by AI?
Back in 2019, at the World Science Congress, Peter van Wijngaarden, Deputy Director of the Center for Eye Research, claimed that the eye sector is one of the leading areas of medicine in terms of artificial intelligence (AI) implementation. According to RANZCO, AI systems are already achieving incredible results and, in some cases, can even rival eye care specialists.
Since AI has become a buzzword, there are hundreds of articles, speeches, and videos on this topic. We are the company that created AI for the eye care and we know the answer to this question: ” Will AI take over optometry and ophthalmology?” ( Spoiler: NO).

Try a co-pilot AI for OCT analysis ( but it won't replace you)
It is simple a misconception. There are a lot of similar examples of AI misconceptions when famous professors and specialists in the field of ophthalmology made predictions that artificial intelligence is rapidly gaining strength in the eyecare industry. This gives rise to many myths and fears around the introduction of AI in clinical practice. Will optometrists be replaced by AI? What about ophthalmologists? What is going on?
The increased attention to the issue of optometrists and ophthalmologists replaced by AI was also provoked by a World Economic Forum (WEF) report. According to this report, people can lose 85 million jobs by 2025 due to the shifting division of labor between people and machines.
In this post, we will discuss the top 5 AI misconceptions that are most often faced by the owners of ophthalmological clinics and optometry centers in order to dispel them once and for all.
What exactly is AI? Do AI algorithms work exactly like a human brain?

The concept of optometrists and ophthalmologists replaced by robots is gaining popularity. Nowadays, eye care specialists often discuss the potential of AI training in human cognitive skills. It is no longer just about the ability of AI to detect Diabetic retinopathy or interpret OCT scans with greater accuracy. The question is, will AI ever be able to replicate human consciousness? And can AI replicate how the human brain works?
What do we know about such models in different areas? AI systems are already demonstrating the work of some human cognitive functions. For example, AI models successfully compete with humans in computer games by gradually learning successful strategies. There is also an AI model which creates enjoyable melodic music.
However, replacing optometrists and ophthalmologists with AI still seems VERY unrealistic. Even with the above examples mimicking some aspects of human behavior, an AI algorithm still needs to learn what empathy is. Artificial intelligence does not understand and cannot make sense of its surroundings, nor can it learn from its surroundings as humans do. The most famous example that confirms this inability of AI is Siri or Alexa. Voice assistants can set up appointments but give strange answers when the conversation goes differently than their scenario.
While the human brain inspires modern AI techniques such as neural networks (NNs), the structure of NNs architectures is not biologically realistic.
First of all, there is a set of qualities that ophthalmologists and optometrists use every day. It is empathy for the patient, as well as creativity, teamwork, and adaptability. These qualities help doctors provide effective care to their patients. It is unlikely that the machines will ever be able to work with children, older adults, or patients with specific disabilities on par with humans. In addition, any patient would like to hear the diagnosis or discuss a treatment plan with a doctor, not a machine.
Therefore AI algorithm can’t work like a human brain, and the scenario where artificial intelligence replace ophthalmologists and optometrists will never happen. Nowadays, there are no developments that would make us think that AI image interpretation will ever be able at least to repeat important qualities of eye care specialists.
Is today’s state of AI dangerous for humans?

Today, AI algorithms can interpret retinal images and distinguish pathological from non-pathological scans. However, not all attempts at AI implementation have succeeded as well. One of the most popular non-medical examples is Facebook. Some time ago, Facebook tried to identify relevant news for certain groups of users. But the automated process could not detect the difference between real and fake news. Russian hackers managed to trick the system and bypass automatic filters. They posted fake news, forcing the Facebook team to come back to human editors.
This is just one example of how security lags behind performance when humans rely on AI too much. Artificial intelligence is a great tool, but in most cases, its abilities only give reliable and the most accurate results in collaboration with eye care professionals. Although machines are designed by humans, they often can’t predict human behavior and don’t know how to cope with situations or clinical cases that go beyond the scope of the algorithm.
Therefore AI is not dangerous for humans when ophthalmologists and optometrists periodically control the work of algorithms and review how the machine works. This is the number-two reason why artificial intelligence replace ophthalmologists and optometrists is unrealistic.
Will AI ever be 100% objective?

To honestly answer the questions of will artificial intelligence replace ophthalmologists and optometrists and whether it is 100% objective, you need to understand that an AI system will only be as good as its inputs. By loading unbiased training datasets, engineers can create an AI system that makes unbiased decisions. However, in the real world, AI is unlikely ever to be 100% objective.
For example, many well-known companies, such as Amazon or Facebook, still struggle with the gender gap in hiring. Some time ago, Amazon used historical data from the past ten years to train its AI recruiting model. The algorithm was supposed to process data and candidates and free recruiters from the routine viewing of hundreds of CVs. However, soon Amazon team discovered that the data was biased against women. AI algorithm was trained by outdated information when the technology industry used to be dominated by men. Thus, the new recruitment system selected only male candidates. This forced Amazon to abandon the algorithm and re-open many recruiter positions.
In the field of ophthalmology, AI models can already accurately predict diabetes risk factors or potential vision loss from OCT images. So when will artificial intelligence replace ophthalmologists? In Altris, we are sure the algorithm will never achieve adequate objectivity, as it will always be limited by input data, whether demographics, gender, or age.
Now we know that AI can’t be 100% objective. Indeed, ophthalmologists and optometrists can’t match the ability of algorithms to detect pixel-level patterns among the millions of pixels in the OCT scan. However, only the cooperation of eye care specialists and a quality AI model working together will allow for more accurate detection of diseases. The combined efforts of AI management systems and eye care specialists can help achieve the desired 100%.
Will optometrists be replaced by AI?What about ophthalmologists?

Various articles have speculated on whether artificial intelligence replace ophthalmologists and optometrists, raising concerns about unemployment. However, this never corresponded to the actual state of affairs. Carl Benedikt Frey, an Oxford Martin Citi Fellow at Oxford University, reported that while 47% of jobs are at risk of automation, the risk for doctors is estimated at only 0.4%.
In addition, in his book “Humans Are Underrated”, Geoff Colvin states that the most valuable skill for ophthalmologists is the ability to sense the thoughts and feelings of patients who are losing sight.
Many patients complain about the lack of contact with the doctor. They admit that the treatment would be more comfortable if doctors devoted more time to live communication. This mainly applies to children and the elderly, who need a lot of attention from eye care specialists. Empathy and similar human qualities are not only an understanding of the patient’s feelings but also an adequate response to them. Thus, a future in which optometrists and ophthalmologists are replaced by AI seems senseless.
Professor Tien Yin Wong, medical director of the Singapore National Eye Centre, claimed that AI holds great promise for retinal screening. And while AI for OCT interpretation will radically change clinical practice, the technology’s more significant impact will be to complement and enhance human capabilities rather than replace them. The field of ophthalmology demonstrates that the combined efforts of scientists and machines are more effective than either could achieve individually.
Artificial intelligence for OCT interpretation is just a recommendation system for an eye care specialist. Often one pathological sign, for example, Cystoid macular edema (CME), or Intraretinal fluid, can indicate many diseases, like Wet AMD, DR, DME, CRVO, and others. That is why AI is only an assistant to a doctor, especially when it comes to rare pathologies.
All in all, AI for OCT interpretation is just a tiny part of clinical practice and can never work without humans. In order to detect the pathological signs and diagnose a disease correctly, an eye care specialist must perform different examination methods. Among these exams are visual acuity, intraocular pressure, ophthalmoscopy, and a basic patient examination, which includes anamnesis. Moreover, ophthalmologists and optometrists may also need to perform other visualization methods, like Fundus photography, FFA, or OCTA.
Will AI replace optometrist?
This is probably one of the key AI misconceptions. Automation has led to a significant change in many industries, and ophthalmology is no exception. So when will AI take over optometry and ophthalmology? The answer is quite simple — AI will never replace eye care specialists. It will eventually take over routine tasks, allowing the careers of ophthalmologists and optometrists to advance in new and exciting directions.

Try a co-pilot AI for OCT analysis ( but it won't replace you)
Automated interpretation of OCT scans will significantly increase the circulation of patients in ophthalmic clinics or optometry centers, which is commercially attractive. Moreover, with increasing life expectancy, and expanding the range and effectiveness of treatment options offered, a collaborative effort between ophthalmologists and AI will improve patient outcomes. This will make ECPs more efficient, freeing up time for human interaction between doctor and patient, which has been a cornerstone of medicine for decades.
There are hundreds of eye care specialists who are already using AIf for OCT scan analysis, for example, to improve! the results. So will AI take over optometry or ophthalmology? The answer is rather simple: No

-

AI for Reading Centers: How it Boosts Workflow and Efficiency
 Mark Braddon
05.10.20227 min read
Mark Braddon
05.10.20227 min readIn recent years reading centers have become an essential resource for facilitating imaging research in many fields, including clinical trials of ophthalmology drugs. And their importance will continue to grow.
Reading centers provide crucial information by evaluating images. That is why for conducting accurate clinical trials, they must hire ophthalmologists of high qualification. Moreover, to ensure consistent analysis, the materials that graders use for the research (be it fundus photographs, fluorescein angiograms, or OCT scans) must also undergo quality control. However, even such measures can’t completely exclude errors or biases.
Meanwhile, recent developments in the field of AI medical image analysis revolutionized the approach to clinical trials, which makes it possible to boost the workflow of reading centers. AI image analysis software works with thousands of images, efficiently providing the large amount of data needed to analyze the patient’s condition. In addition, evaluating images with AI is faster, cheaper, and more effective.

Book demo for a company
Try artificial intelligence for OCT analysis
In this article, we will discuss the top 5 benefits of AI medical image analysis software for reading centers and the way AI improves the image interpretation process.
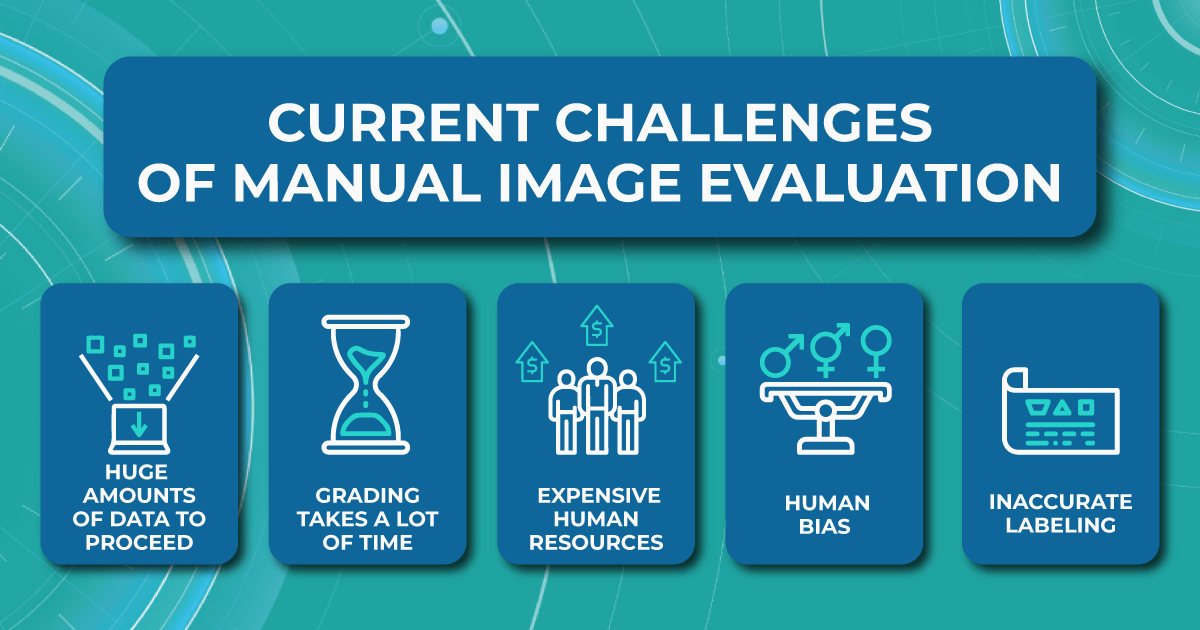
Limitations of the manual evaluating procedure
Although several reading centers have already implemented AI for medical image analysis in their workflow, most organizations are far from evaluating automation and prefer classic image interpretation methods.

In most reading centers, ophthalmologists manually evaluate ocular images for drug safety studies, compile the images, and perform statistical analysis of the data. Research sizes for reading centers can range from 50 images to 3000 or more, and dozen of separate sets of images can be collected per research subject. Therefore reading centers have many obstacles to a quality evaluation process and accurate results.
-
Large amount of images is hard to proceed
The vast number of images that need to be processed in the short term usually leads to the main problem for reading centers — most hire outsourced ophthalmologists to speed up the image grading and evaluation process. Outsourced specialists have different levels of qualification and different evaluating methods, which may lead to decreased accuracy. In addition, outsourced eye care specialists are not always interested in performing the work at the highest level.
-
Human resources are expensive
Another limitation of the standard evaluating procedure is the high сost spent on ophthalmologists. Human resources are usually quite expensive and associated with the risk of staff turnover.
-
High probability of human bias
Besides, hours spent in front of a computer screen evaluating thousands of images create a stressful environment for ophthalmologists and cause many errors, affecting the accuracy of the clinical trials. Even the FDA recognizes grader fatigue and its impact on potential errors in image interpretation.
-
Inaccurate labeling
In addition, administrative problems also occur quite often. This happens due to deviations from study protocols and incorrect labeling of images, which can compromise the integrity of the analyses.
Fortunately, the pace of digitalization in reading centers is accelerating. Here is how AI medical image analysis can help reading centers cope with the growing workload.
The importance of implementing AI medical image analysis for reading centers
Usually, AI image analysis is made through a pattern recognition process that involves scanning images for specific pathological signs to interpret the patient’s condition. The AI image analysis software has precise and efficient evaluation protocols that allow the analysis and interpretation of images in terms of a variety of qualitative morphological parameters. For example, when analyzing images of a patient with diabetic retinopathy, the AI models recognize microaneurysms or hemorrhages.

AI algorithms allow reading centers to conduct trials of any size and duration, including various treatments for various eye diseases. Moreover, unlike the standard image interpretation process, which requires significant human resources, the introduction of AI for image analysis into the workflow of reading centers has many advantages.
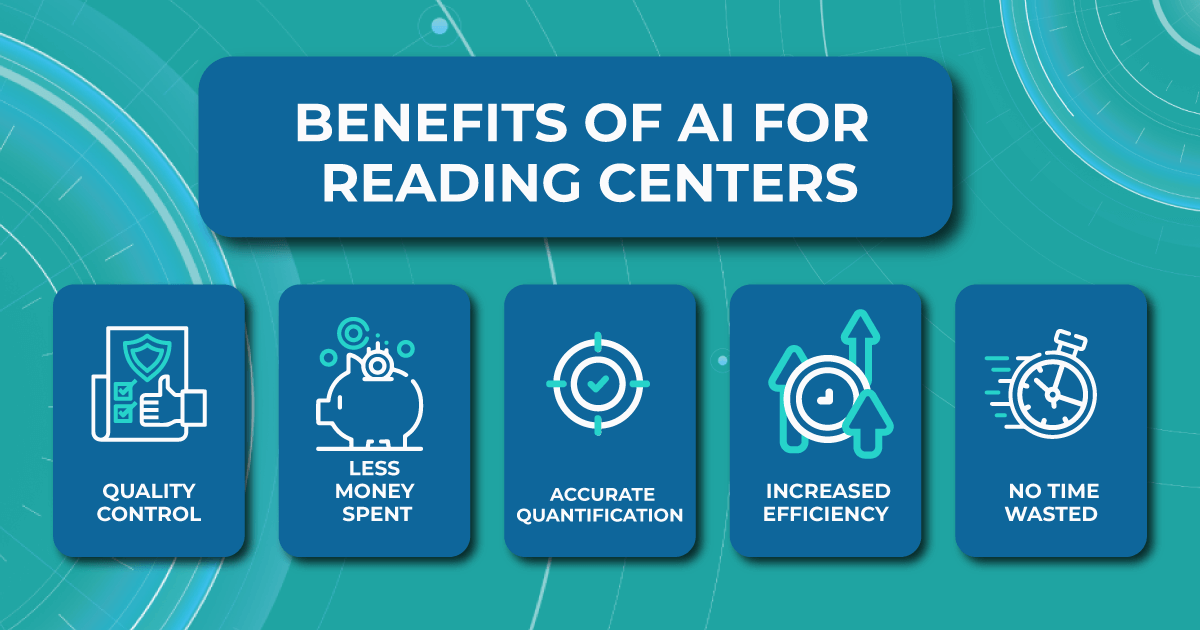
- Quality control. Using AI algorithms ensures no errors in OCT scan analysis. AI image analysis software ensures that the desired parameters are classified based on certified imaging protocols.
- Less money spent. Implementing AI-assisted OCT analysis is less expensive than hiring outsourced ophthalmologists.
- Accurate quantification. AI in medical image analysis does not depend on patient characteristics or treatment group assignment knowledge, so the machine provides the most objective and accurate assessment possible.
- Increased efficiency. Improving the reading centers workflow with AI provides an objective and standardized classification of images. It means that any human bias is excluded, which increases the reputation of clinical research.
- No time wasted — no more hours spent at a computer screen. Evaluating images with AI medical image analysis provides faster and more sensitive identification of the patient’s condition, which can positively impact decision-making.

Book demo for a company
Try artificial intelligence for OCT analysis
How reading centers will benefit from AI image analysis software
In short, image evaluation with algorithms is fast, less expensive, and more reproducible. However, many companies that perform clinical trials in cooperation with reading centers are still afraid of implementing AI in medical image analysis and evaluating processes. Modern AI-based image management systems, such as Altris AI, unlike their predecessors, allow reading centers to overcome the challenges of the manual image interpretation process.
A lot of data available to train an algorithm
The more images with various pathological features the algorithm has for training, the more accurately it will detect the diagnosis. Modern AI image analysis software has the ability to obtain thousands of OCT images from different models of devices for comprehensive and correct training of algorithms. Although many medical centers keep their clinical practice confidential, many ophthalmic cases and images with various pathological signs in the public domain allow the training of AI algorithms.
For example, the Altris AI medical image analysis software was trained on 5 million unique OCT scans obtained in 11 practicing ophthalmology clinics through the years. Our retina experts took a responsible approach to annotating and labeling images for algorithm training. A thorough error detection and correction procedure gave our algorithm 91% accuracy.
Constant quality control
The responsibilities of the modern algorithms developers include not only the release of the model but also further diagnostics, which allows avoiding the problem of reproducibility. After all, constant quality control is necessary for algorithm development environments. Understanding the importance of quality control, the Altris AI team constantly tests the reproducibility of AI medical image analysis model diagnostics.
Collection of rare diseases
According to our research, 25% of ophthalmologists, on average, miss rare pathologies 3 times a week. However, modern AI image analysis software allows overcoming this challenge. For example, Altris AI excludes missing minor, early, rare pathologies. Our team created an algorithm that automates the detection of 54 pathological signs and 49 pathologies.
High percentage of algorithmic bias is avoided
Algorithmic bias is one of the biggest challenges in AI. Although algorithms themselves do not have biases, they inherit them from humans. However, today, AI for image analysis has learned how to overcome the lack of interoperability between medical record systems.
Although it is impossible to avoid algorithmic bias completely, as it can appear at any stage of the algorithm creation process, from study design and data collection to algorithm development and model selection, modern developers take a direction to fair AI. By using a technical and regulatory framework that provides the diverse data needed to train AI algorithms, the Altris AI team makes modern technologies inclusive and ensures algorithmic bias can be excluded.
The future of AI medical image analysis in reading centers
The ultimate goal of the ophthalmic AI system for reading centers is to improve the grading and evaluating process and obtain more accurate research results. However, instead of fully digitalizing image assessment, the ideal approach to analysis is integration — where the benefits of AI algorithms and human skills can be combined.
Technology will never fully replace humans, but it is already improving their work efficiency. For example, by taking over more routine and monotonous tasks, algorithms allow ophthalmologists to focus on specific eye areas and increase the evaluation speed. AI medical image analysis software can also be effective in determining compliance with the standardization of feature interpretation and determining image quality for requesting more images.
There are undoubtedly many challenges to integrating AI for image analysis into the workflow of reading centers. However, modern AI technologies can already overcome almost all of them. Altris AI image interpretation system is changing the future of clinical research by helping to classify images faster and increasing the efficiency, accuracy, and reproducibility of clinical trial data.
You can watch a short video of how Altris AI platform assists eye care specialists in detecting pathological signs on the OCT scans:
-
-

The Role of AI Image Interpretation for Ocular Pathologies Detection
 Maria Znamenska
28.09.202220 min read
Maria Znamenska
28.09.202220 min readThe burden of timely diagnostics lies on the shoulders of eye care specialists: ophthalmologists and optometrists worldwide. According to the International Agency for the Prevention of Blindness, over 1 billion people live with preventable blindness because they can’t access the proper diagnostics and treatment. Almost everyone needs access to eye care services during their lifetime. Unfortunately, there are only 331K optometrists worldwide, while 14M optometrists are required to provide effective and adequate eye care services.
With the high prevalence of the population that needs eye care services and the lack of specialists, the goal of timely and accurate diagnostics and treatment seems unachievable.

See how AI for OCT works
However, the empowerment of eye care specialists with Artificial Intelligence (AI) can be a real solution to this problem. As the larger part of the work of eye care specialists relies on retina image assessment and analysis, the support of this process can unburden ophthalmologists and optometrists all over the world. Modern AI image interpretation algorithms, such as Altris AI, can discover patterns among millions of pixels with high speed, accuracy, and zero human errors because of tiredness.
You can watch a short video of how Altris AI can assist you in detecting pathological signs on the OCT scans:
https://www.youtube.com/watch?v=Ehhwl6Q0O-A&ab_channel=Altris
In this article, we will talk about the capabilities of AI image interpretation for Optical Coherence Tomography (OCT) in detecting common pathologies, such as AMD or glaucoma, and less prevalent, such as Choroidal Melanoma. Despite the skepticism of the eye care community towards AI, multiple research works mentioned in this article prove the efficiency of AI. Moreover, there are market tools, capable of detecting 49 eye pathologies with 91% accumulative accuracy. Altris, the SaaS created by a team of retina experts based on 5 million OCT scans obtained in 11 clinics, is such a tool.
AI image interpretation for OCT
AI image interpretation for Asteroid Hyalosis
Asteroid hyalosis is a clinical condition in which calcium-lipid complexes are suspended throughout the vitreous collagen fibrils. Although it is a rare disease (1.2% prevalence according to the U.S. Beaver Dam Eye Study), it also may lead to unpleasant consequences, such as surface calcifications of intraocular lenses. Today OCT can help with the detection of this degenerative condition. For a higher confidence level, eye care specialists may use Altris AI image interpretation for OCT analysis to detect asteroid hyalosis.
AI for Central Retinal Artery Occlusion (CRAO)
Central Retinal Artery Occlusion (CRAO) presents as unilateral, acute, persistent, painless vision loss. It can be bilateral in 2% of the population. The vision loss is abrupt, and the treatment is only effective during the first hours. CRAO resembles a cerebral stroke. Therefore, its treatment should be similar to any acute event treatment: detecting the occlusion site and ensuring it won’t occur again. AI image interpretation models, such as Altris AI, can assist eye care specialists in detecting CRAO today.
AI for Central Retinal Vein Occlusion (RVO)

CRVO is one of the most widespread vascular diseases that affect the population over 45. There are two distinct types of CRVO: perfused (nonischemic) and nonperfused (ischemic). Each of these types has its symptoms and treatment prognosis. For instance, ischemic CRVO leads to sudden visual impairment, while nonischemic CRVO development takes time to develop mildly. The detection of CRVO is now done with the help of OCT predominately, and AI image interpretation systems shows promising results in spotting its symptoms, such as nonperfusion. Altris AI system defines CRVO with 91+% accumulative accuracy in detecting pathological signs that indicate the CRVO.
AI for Central Serous Chorioretinopathy (CSC)
Accumulation of fluid under the central retina is called central serous chorioretinopathy. Over time, this disease can lead to the distortion of vision. Fortunately, available AI models for OCT scan analysis show high accuracy in detecting CSC. This and other AI image interpretation models effectively discriminate between acute and chronic CSC, and their performance can be comparable to the performance of ophthalmologists. Altris AI is already helping eye care specialists worldwide to diagnose CSC cases.

Try Altris AI for free
Check how artificial intelligence assists in OCT interpretation
AI for Chorioretinal Scar
Chorioretinal scars are tiny scars in the back of the eye, the size of which may vary from 0,5mm to 2mm. In most cases, the chorioretinal scar appears as the result of virus infection, such as toxoplasmosis and toxocariasis, or trauma. It usually has no malignant potential. Modern AI image interpretation algorithms allow ophthalmologists and optometrists to diagnose chorioretinal scars more accurately by relying on OCT images.
AI image interpretation for Chorioretinitis
The inflammation of the choroid is called chorioretinitis. Often, the inflammatory process can be caused by congenital viral, bacterial, or protozoan infections. Chorioretinitis is characterized by vitreous haze, fine punctate gray to yellow exudation areas, pigment accumulation along the optic nerve and blood vessels, and flame-shaped hemorrhages with chorioretinal edema. The goal of the eye care specialist is to detect chorioretinitis which can potentially lead to blindness, and to eliminate inflammation. Altris AI image interpretation system can be an excellent decision-making support tool in detecting chorioretinitis.
AI image interpretation for Choroidal Melanoma
Today, choroidal melanoma is the second most common intraocular tumor in the adult population. Patients with choroidal melanoma don’t have distinct symptoms but can have impaired visual acuity, visual field defects (scotomas), metamorphopsia, photopsia, and floaters.
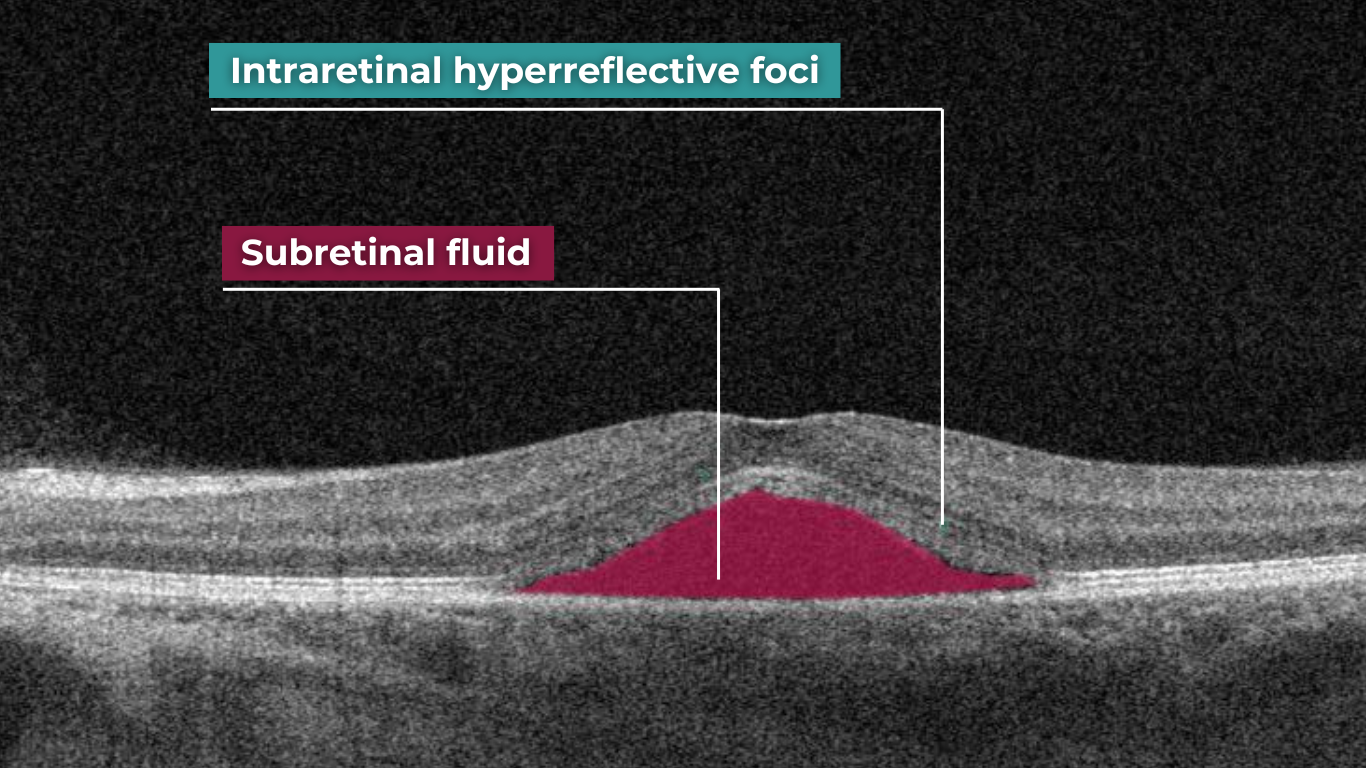
OCT is a relatively new method for the detection of choroidal melanoma, which is nevertheless gaining popularity. OCT cannot be the only diagnostic method for melanoma detection – FA is also needed for final diagnosis. However, optical shadowing, thinning of overlying choriocapillaris, subretinal fluid, retina local elevation, subretinal lipofuscin deposits, and disrupted photoreceptors can be detected with the help of OCT.
Such pathological signs will indicate possible choroidal melanoma. Altris AI image interpretation system can assist eye care specialists with detecting pathological b-scans and locating this disease.
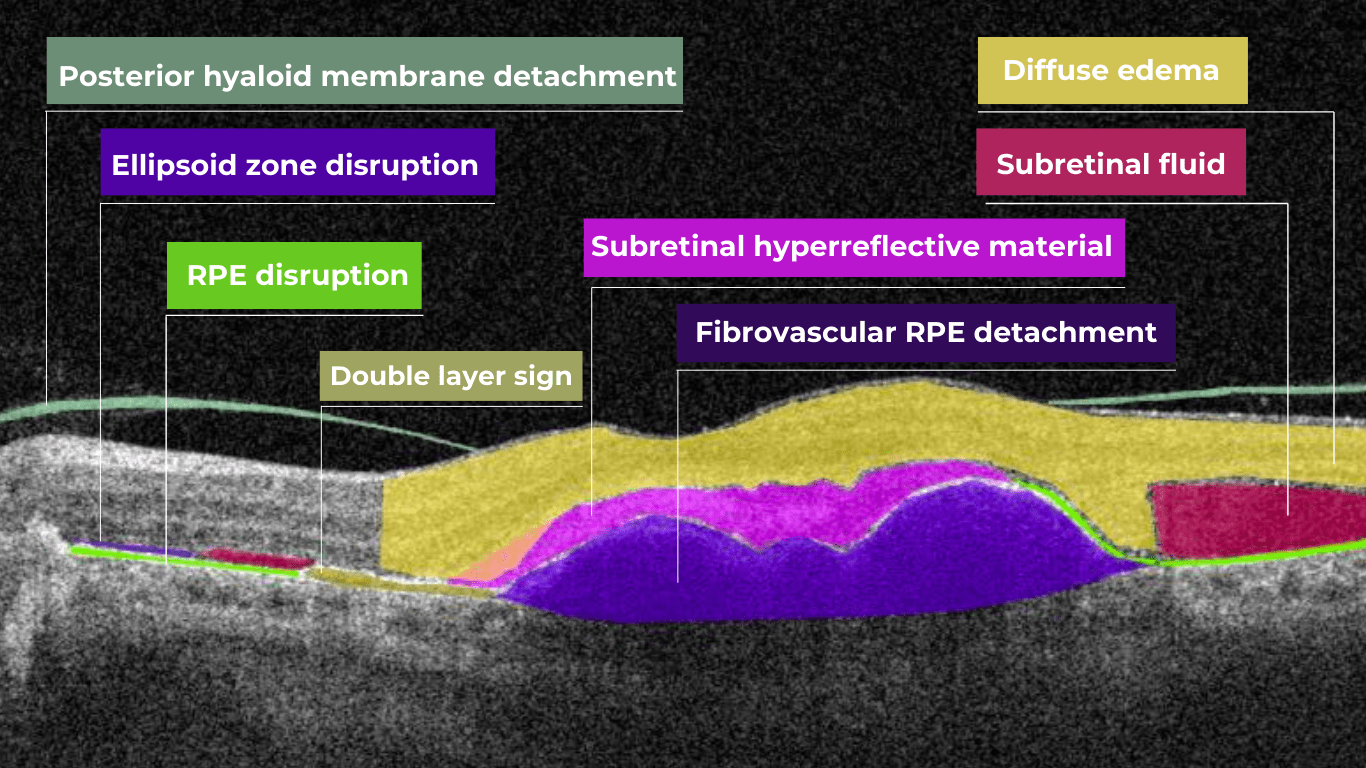
AI for Choroidal Neovascularization (CNV)

Choroidal neovascularization (CNV) is part of the spectrum of exudative age-related macular degeneration (AMD) and some other conditions. CNV is an abnormal growth of vessels from the choroidal vasculature to the neurosensory retina through Bruch’s membrane.
Modern OCT systems can detect even a tiny amount of fluid leaking into the retina. Empowered by Altris AI image interpretation algorithm, eye care specialists can spot pathological signs of choroidal neovascularization much faster or detect the pathologies that accompany CNV, resulting in better patient outcomes.
AI image interpretation for Choroidal Rupture
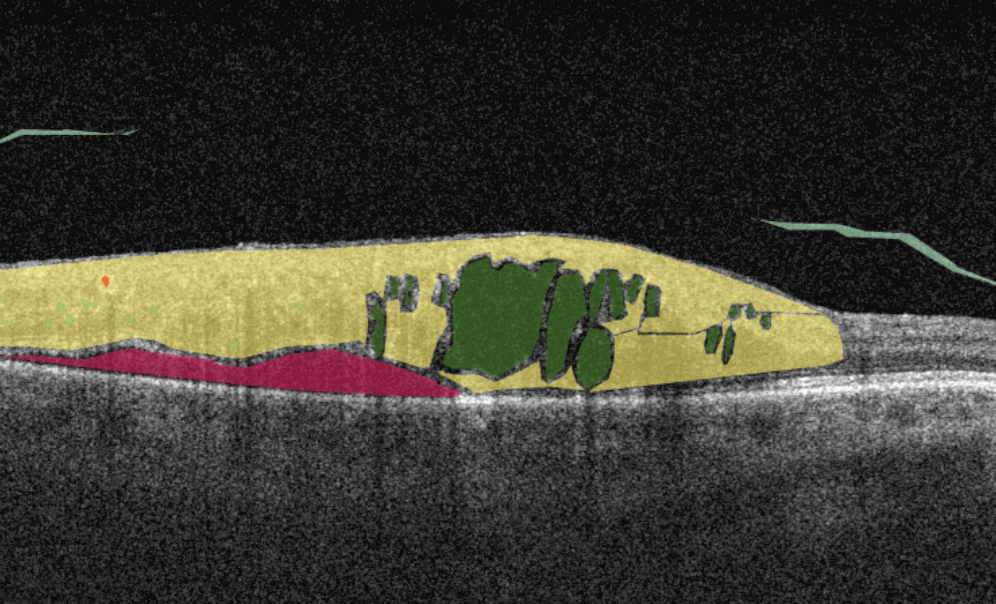
Traumatic choroidal rupture is common after blunt ocular trauma (5 to 10%). It is a defect in the Bruch membrane, the choroid, and the retinal pigment epithelium. The location of the choroidal rupture will define the symptoms: if the fovea and parafoveal retina are included in the rupture area, patients experience impaired vision. In other cases, the rupture can be asymptomatic. OCT is used to diagnose choroidal rupture as it can show the loss of continuity of the RPE layer and the thinning of the choroid. AI image interpretation is exceptionally accurate in layers segmentation and volume/area calculation, so missing the symptom of choroidal rupture with AI is almost impossible.
AI image interpretation for Choroidal Nevus

Choroidal nevus is a benign melanocytic tumor of the choroid and is found in 5 to 30% of white people. It can be found accidentally because it is asymptomatic. Artificial intelligence methods are used not only for identifying choroidal nevus but also for early signs of its transformation into malignant melanoma. The earlier the small melanoma is detected, the better the treatment prognosis is for the patient. Altris AI image interpretation system is one of the systems capable of detecting choroidal nevus before its transformation into melanoma.
AI for Cone-Rod Dystrophy (CORD)
CORD is an inherited retinal disease caused by a genetic mutation characterized by cone photoreceptor degeneration. It may be followed by subsequent rod photoreceptor loss. CORD symptoms include loss of central vision, photophobia, and progressive loss of colored vision. OCT diagnostics help to diagnose CORD by pointing at the absent interdigitation zone and progressive disruption and loss of the ellipsoid zone (EZ). Today AI image interpretation is helping to detect Cone-Rod Dystropthy to eye care specialists more confidently, even in controversial cases.
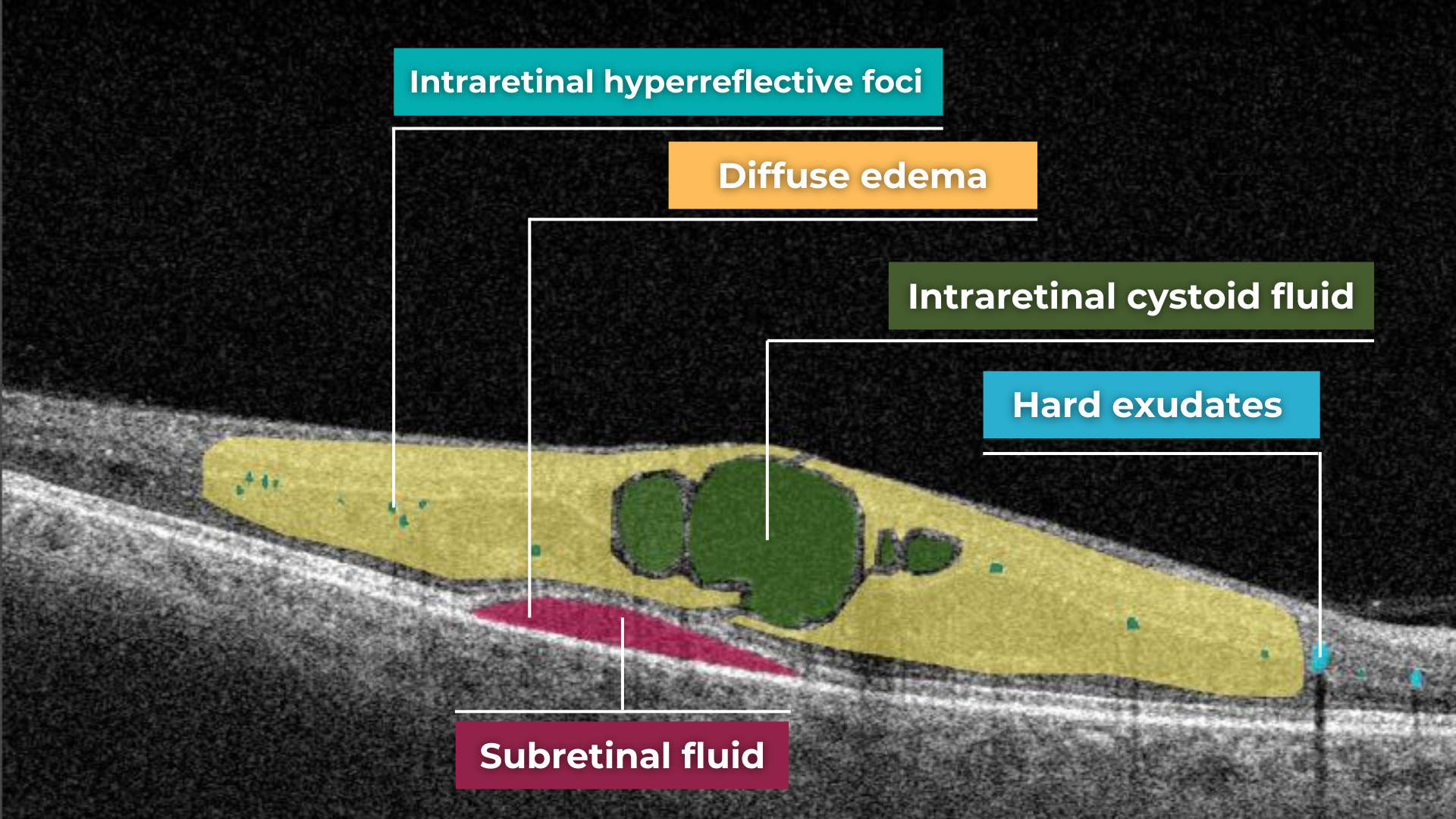
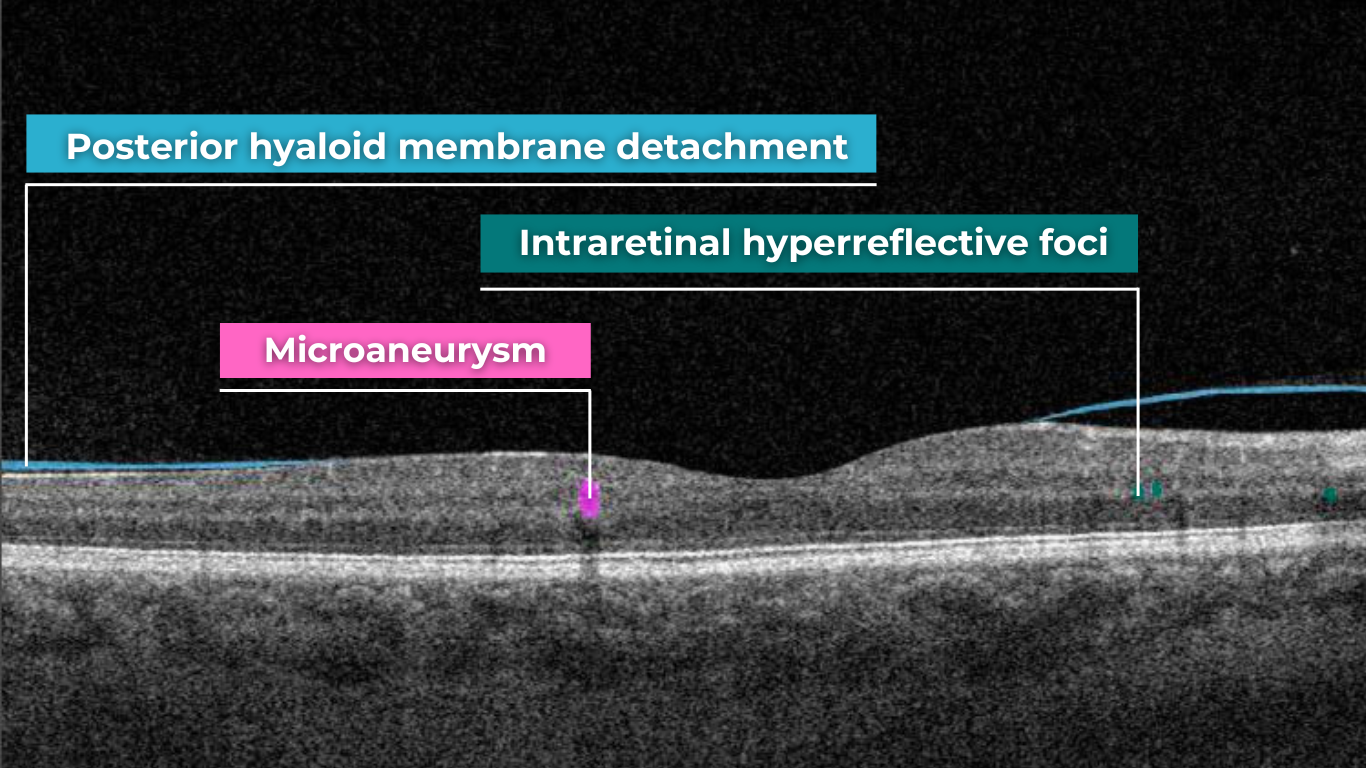
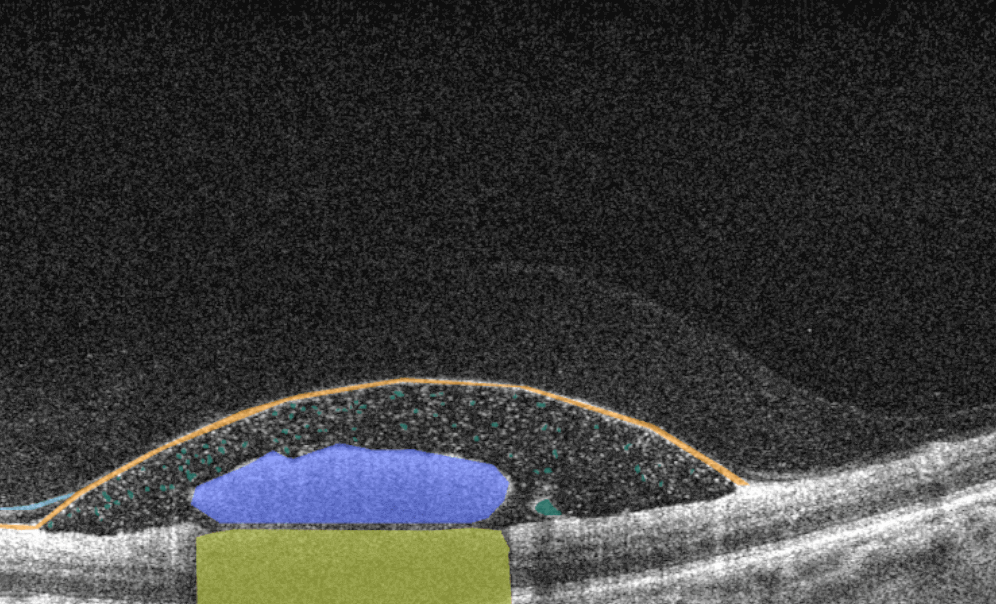
AI for Cystoid Macular Edema (СME)

Cystoid macular edema (CME) is a painless condition in which cystic swelling or thickening occurs of the central retina (macula) and is usually associated with blurred or distorted vision. CME can be caused by many factors, including diabetic retinopathy and age-related macular degeneration (AMD). OCT diagnostics help to spot СME by detecting retinal thickening with the depiction of the intraretinal cystic areas. CME is not irreversible. Vision loss caused by macular edema can be reversed if detected early. Combining OCT diagnostics with AI image interpretation, eye care specialists can detect CME with higher accuracy at an earlier stage.
AI image interpretation for Degenerative Myopia

Degenerative or pathological myopia is the condition during which axial lengthening occurs, especially in the posterior pole. It leads to retina stretching, the sclera’s thinning, choroidal degeneration, and potential loss of vision. AI image interpretation systems have demonstrated excellent results in detecting pathologic myopia and identifying myopia-associated complications on OCT. AI helps ophthalmologists improve the monitoring of pathology treatment and classify different cases of myopia.
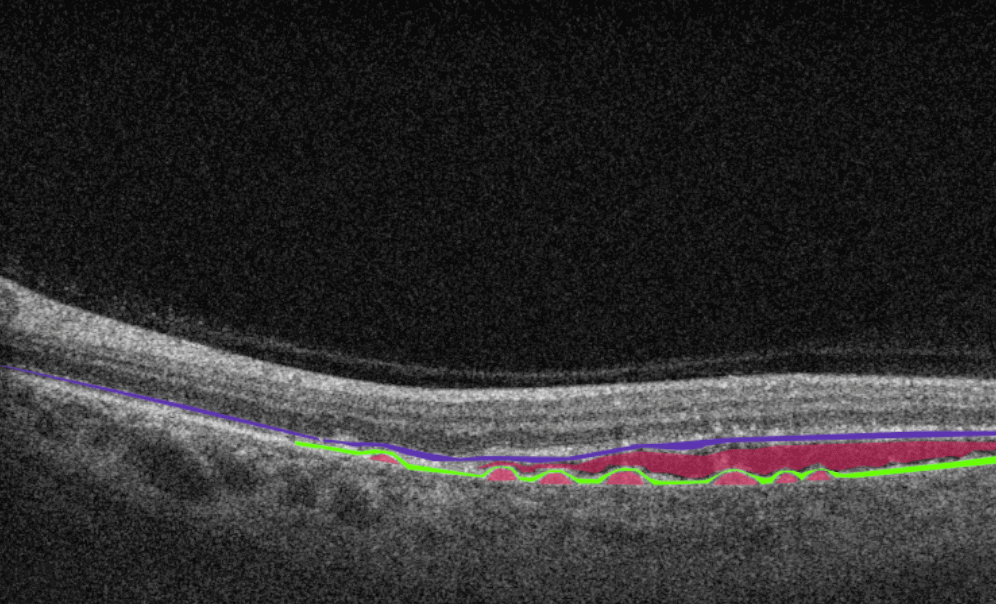
AI for Diabetic Macular Edema
Diabetic macular edema (DME) is the presence of excess fluid in the extracellular space within the retina in the macular area, typically in the inner nuclear, outer plexiform, Henle’s fiber layer, and subretinal space. DME can develop during any stage of diabetic retinopathy in patients with diabetes.
Unfortunately, the early symptoms of DME can be unnoticeable or include impaired vision and reading and color perception problems, which some people may ignore. Taking into account its asymptomatic nature, patients with diabetes need regular OCT examinations to determine the presence of DME. OCT has become a golden standard in DME detection within the last few years, and AI can be an excellent decision-making support tool in OCT scans interpretation. According to recent research, AI-powered OCT analysis provides an accurate diagnosis of DME with a cumulative accuracy of over 92%.
AI image interpretation systems can unburden ophthalmologists and optometrists who have a lot of patients due to their convenience and can be used in remote regions of the world in the future.
AI image interpretation for Diabetic Retinopathy

Diabetes can affect the eyes in various ways, most commonly corneal abnormalities, glaucoma, iris neovascularization, cataracts, and neuropathies. However, diabetic retinopathy (DR) is the most common and potentially the most blinding of these complications. Early treatment of both proliferative and non-proliferative DR can improve patient outcomes significantly. OCT is a common diagnostic method for diabetic retinopathy. It relies on the localization of intraretinal and/or subretinal fluid and can help to diagnose diabetic retinopathy through pathological signs detection and layer thickness measurement.
AI image interpretation is a step in the future of detection that shows high sensitivity in identifying DR, and studies prove its effectiveness. AI-assisted analysis of OCT scans helps eye care specialists today and will definitely be more widespread tomorrow.
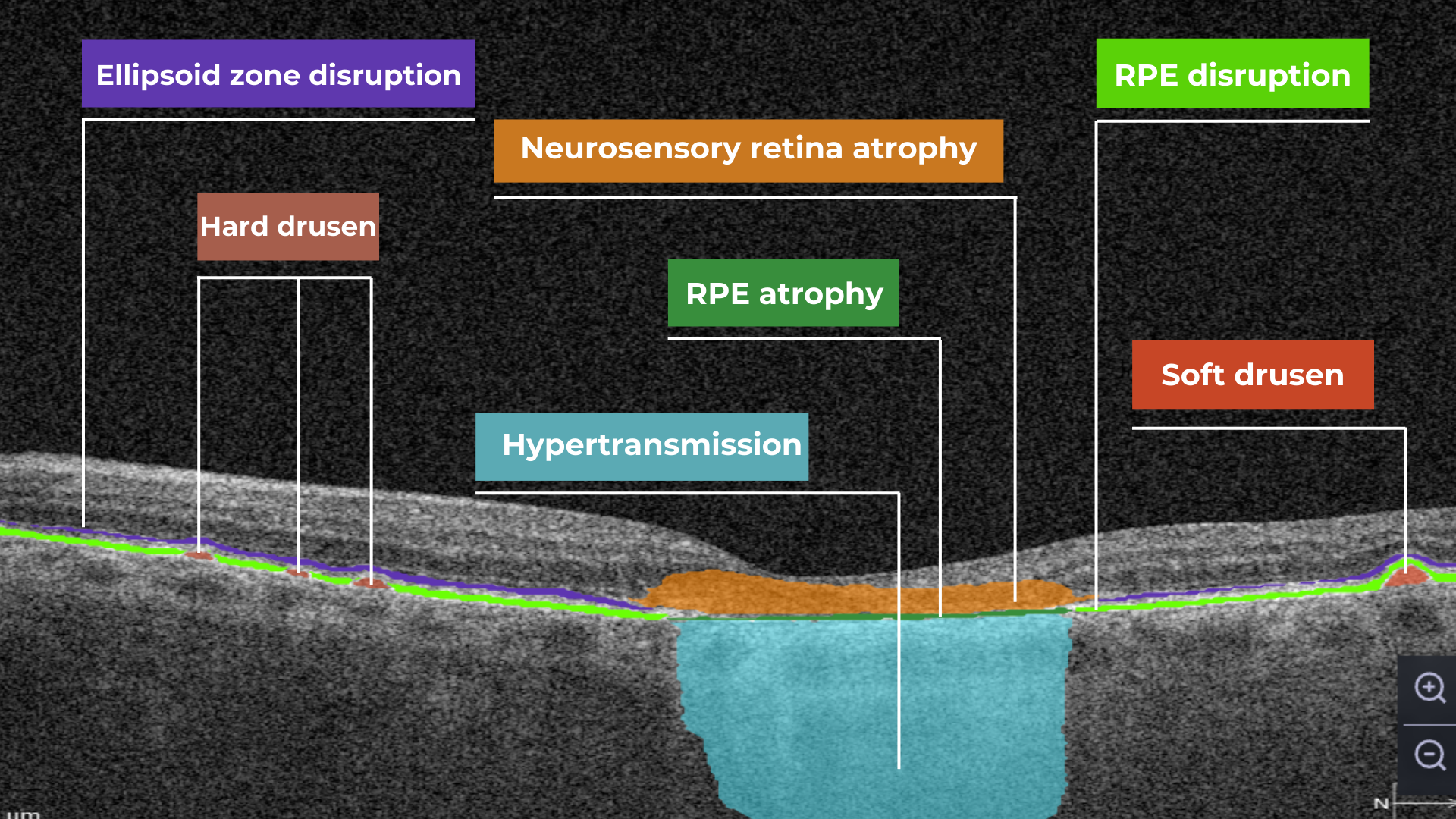
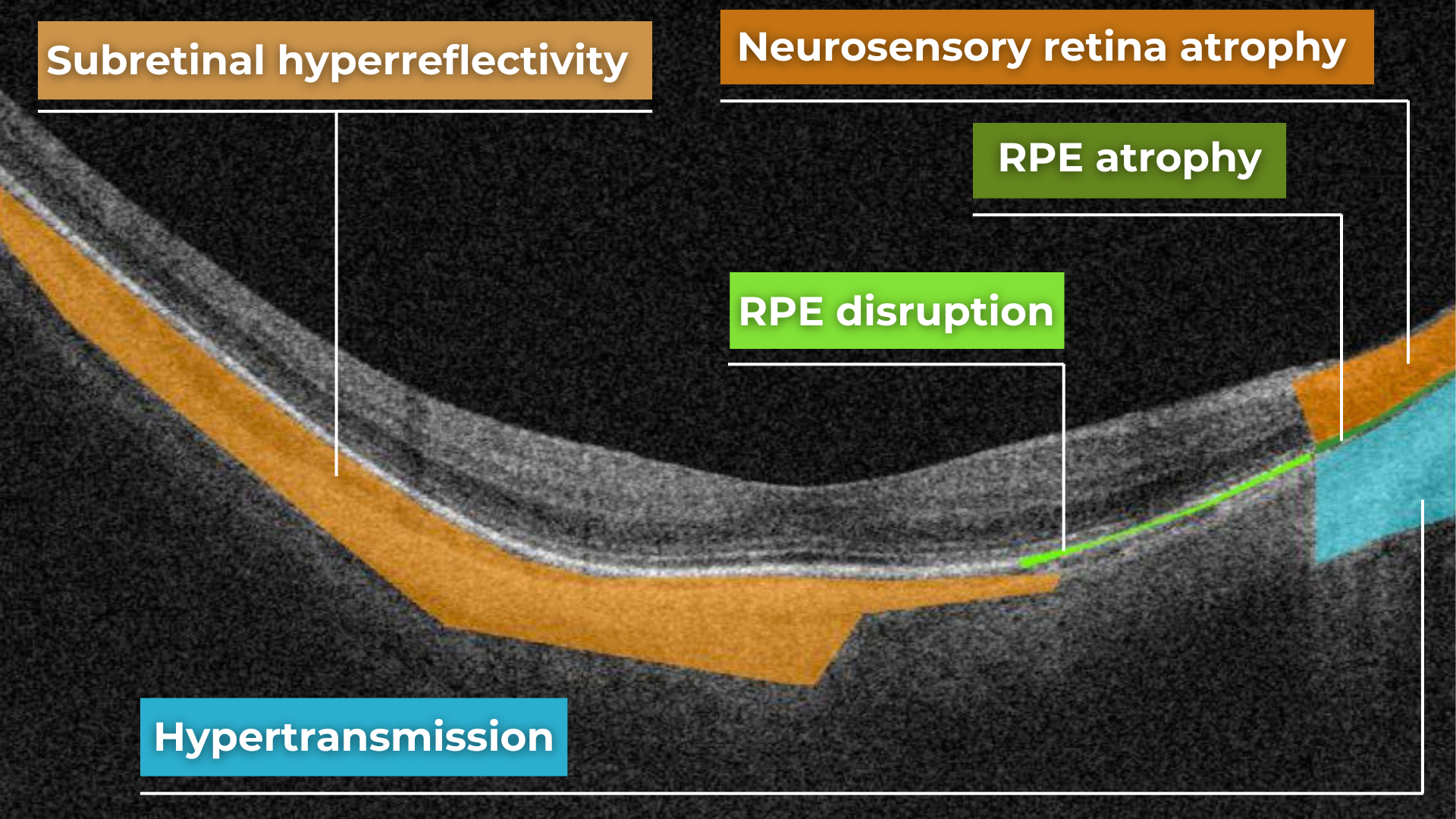
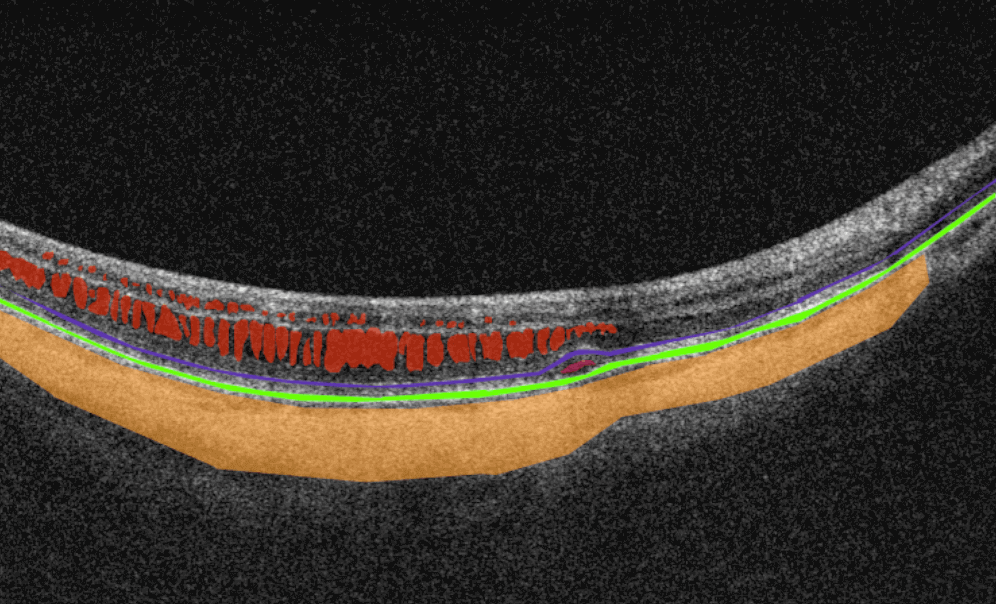
AI image interpretation for Dry AMD
Dry AMD is a more common type of AMD (80% of people have this type), during which patients slowly lose their central vision. It is the aging of the macula and the appearance of deposits called drusen. There is no treatment for Dry AMD yet. However, early detection can help patients to change their lifestyles and slow down the development of this disease. Modern AI solutions make it possible to diagnose Dry AMD faster and develop successful methods of treating the disease. AI image interpretation systems also exclude the possibility of human error.
AI for Dry AMD – Geographic Atrophy
Geographic atrophy is an advanced form of the late stage of Dry AMD development. In this condition, retina cells will degenerate and finally die, leading to the patient’s central vision loss.
AI image interpretation is being widely used to detect Geographic Atrophy with the help of OCT. In this meta research, there are numerous studies that focus on lesion segmentation, detection, and classification of geographic atrophy and even its prediction. They vary in accuracy, but the overall trend of AI for geographic atrophy detection is very positive. The use of artificial intelligence has several advantages, including improved diagnostic accuracy and higher processing speed.
AI for ERM or Epiretinal Fibrosis

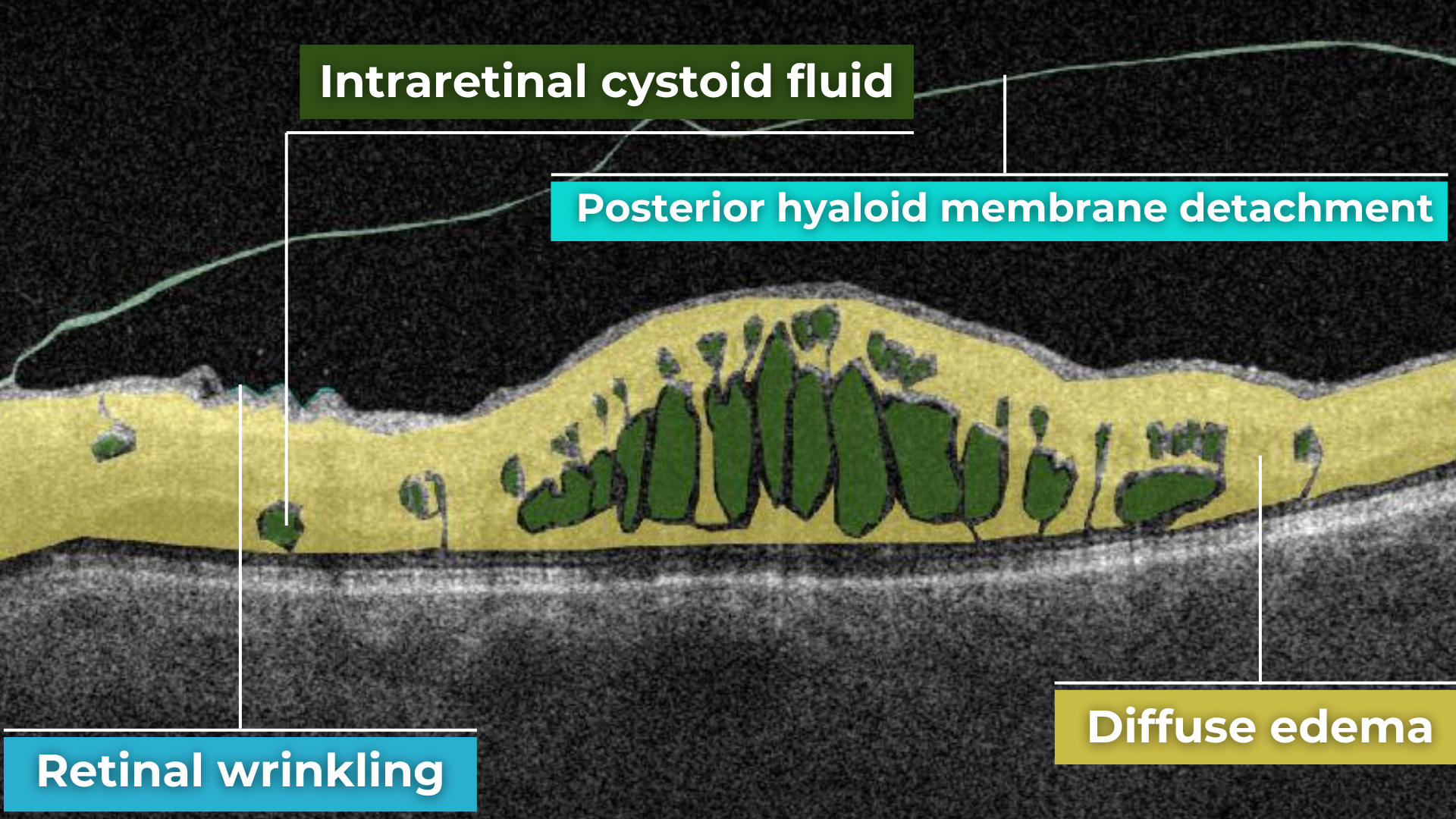
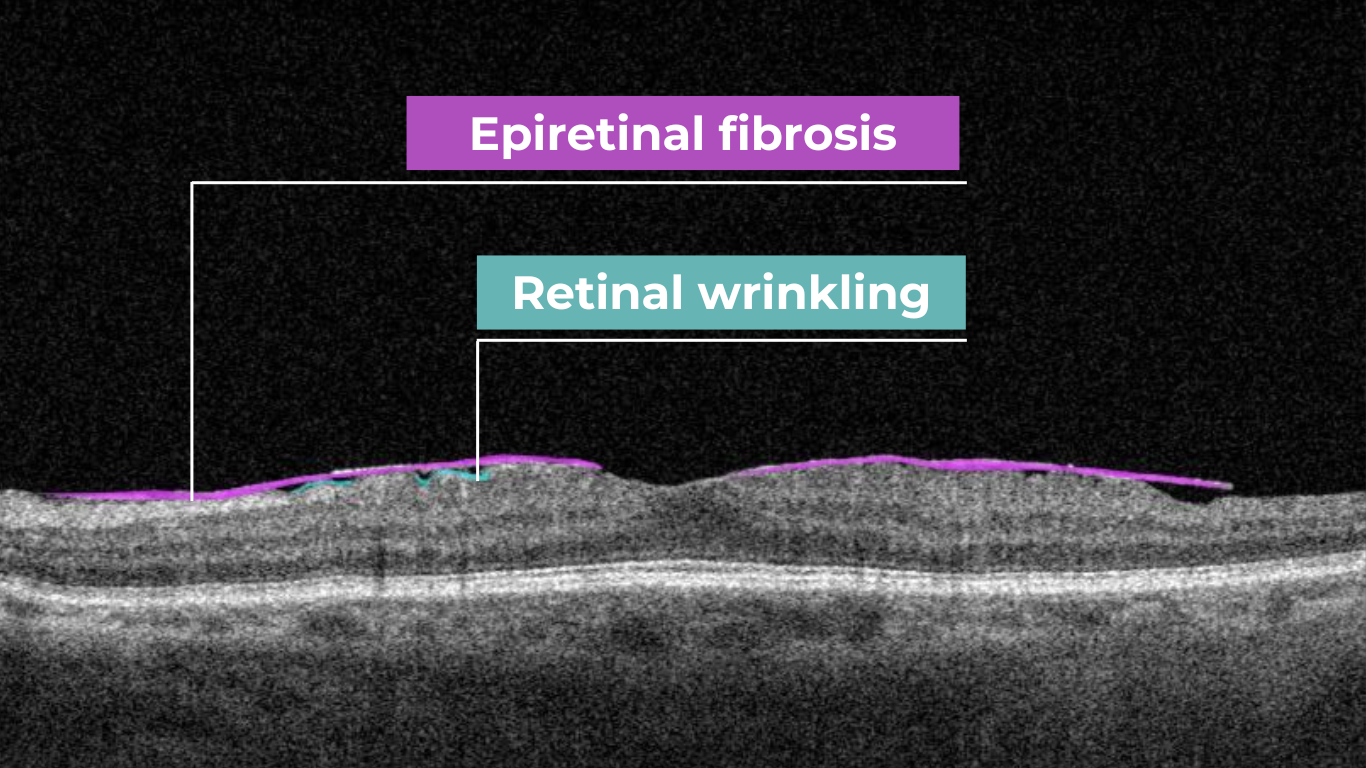
Epiretinal fibrosis (epiretinal membrane or macular puckering) is a treatable cause of visual impairment. It is a macula disease caused by fibrous tissue growth on the retina surface. AI image interpretation model for detecting ERM on OCT can outperform non-retinal eye care specialists with a cumulative accuracy of 98+%. For more professional retina experts, AI can be a decision-support tool. Early detection and treatment of this disease are crucial to prevent the growth of fibrous tissue and the worsening of the patient’s condition.
AI image interpretation for Epiretinal Hemorrhage
Epiretinal hemorrhages result from a serious trauma: car or sports accidents, falls, and direct physical impact. Mild hemorrhages unrelated to a serious traumatic event can disappear on their own, but they can be a symptom of a more complex pathology. Epiretinal hemorrhages can be detected with the help of OCT, and AI image interpretation systems can make this process more accurate.
AI for MTM (Foveoschisis)
Myopic foveoschisis or myopic traction maculopathy is the thickening of the retina that reminds schisis in patients with high myopia with posterior staphyloma.
Untreated foveoschisis often leads to vision loss due to secondary complications, which is why this disease should be detected in time. Today when OCT is becoming more widespread, detection of foveoschisis is more common and accurate. More than that, the studies show that combining the power of AI image interpretation and OCT diagnostics for MTM detection is equal to the junior ophthalmologist’s knowledge. Using AI-powered OCT, it is possible to deal with the shortage of specialists that can guarantee timely diagnostics.
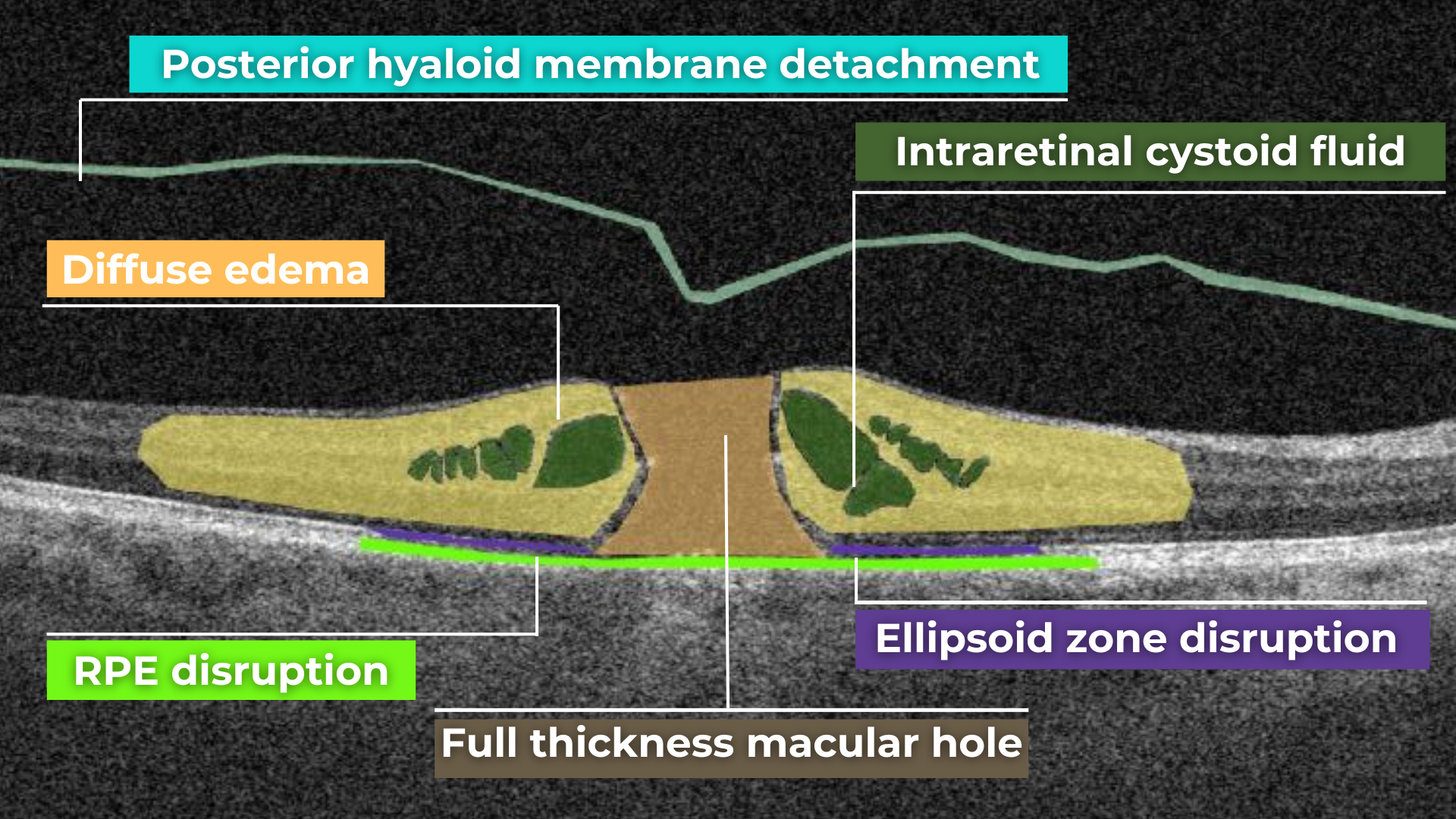
AI for Full-thickness Macular Hole

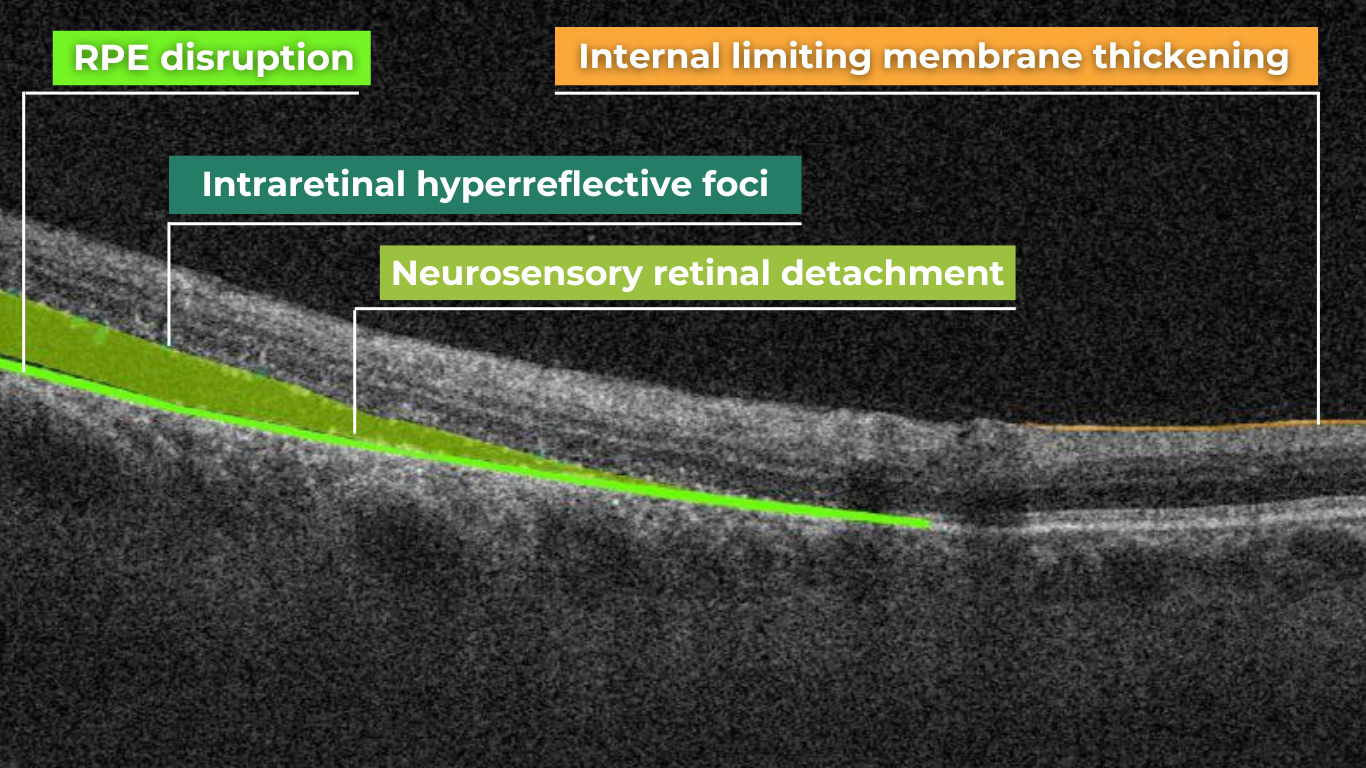
A macular hole is a full-thickness defect of the retina involving the foveal region. Patients usually present a reduction of central visual acuity. A complete ophthalmic examination, including OCT, should be performed to diagnose a full-thickness macular hole. So far, the research of AI image interpretation algorithms for a full-thickness macular hole is dedicated to OCT(A), but there are available tools on the market that can help define full-thickness macular hole on OCT scans as well. Altris AI is one of them.
AI for Hypertensive Retinopathy
People with high blood pressure, older people, and patients with diabetes often develop hypertensive retinopathy. OCT examination can be used for the detection of hypertensive retinopathy.
AI-based OCT analysis shows promising results in detecting hypertensive retinopathy by defining retinal vessels and other pathological signs in the retina.
AI for Intraretinal Hemorrhage

Among patients with DR, RVO, or ocular ischemic syndrome, there are often those who develop side pathologies. One of these pathologies is intraretinal hemorrhage. AI image interpretation systems help ophthalmologists and optometrists identify intraretinal hemorrhages in the retina.
AI image interpretation for Vitreous Hemorrhage
Vitreous hemorrhage results from bleeding into one of the several potential spaces formed around and within the vitreous body. This condition can follow injuries to the retina and uveal tract and their associated vascular structures. Eye care specialists should perform a complete eye examination, including OCT, slit lamp examination, intraocular pressure measurement, and dilated fundus evaluation. Timely diagnosis and treatment are essential: it can significantly reduce concomitant diseases of intravitreal hemorrhage. AI image interpretation systems can help eye care specialists detect vitreous hemorrhage supporting them in case of controversial OCT scans.
AI for Lamellar Macular Hole (LMH)

Lamellar macular hole is one of the types of macular holes known in eye care practice. The problem is that the stage 0 macular hole is a clinically silent finding detected on OCT where a parafoveal posterior hyaloid separation is present and a minimally reflective preretinal band is obliquely inserted at one end of the fovea. Eye care specialists may have problems identifying lamellar macular hole on OCT. That is where AI image interpretation models can come into play.
AI for Laser-induced Maculopathy
Since 2014, the number of laser injuries reported worldwide has more than doubled because of the widespread use of laser technologies. Depending on the damage, the patient may have a quick recovery or long-term vision loss with the development of diseases such as photoreceptor’s damage, macular hole, ERM, or others. OCT is one of the methods that help to detect laser-induced maculopathy without human errors and doubts. AI image interpretation models have a reasonable prospect of helping eye care specialists define laser-induced maculopathy based on OCT scans.
AI for Age-related Macular Degeneration (ARMD)
Age-related macular degeneration is one of the leading reasons for blindness in people of older age, especially among women and people with obesity. Patients usually present with a gradual, painless vision loss associated with delayed dark adaptation, severe metamorphopsia, and field loss. In other words, in the early stages of AMD, patients may not have any signs or symptoms, so they may not even know they have the disease. Regular OCT screening (among other diagnostic methods) can be a life-saving vest for older people.
AI image interpretation has shown great promise in detecting AMD, and research papers show that its capabilities are similar to those of ophthalmologists. AI-powered automated tools provide significant benefits for AMD screening and diagnosis.
AI for Macular Telangiectasia Type 2
Macular telangiectasia (Mac Tel) results from the capillaries abnormalities of the fovea or perifoveal region related to the retina nuclear layers and ellipsoid zone.
Macular Telangiectasia Type 2 can have negative consequences and develop into cystic cavitation-like changes in all the layers of the retina or even transform into a full-thickness macular hole. OCT is an effective diagnostic method of macular telangiectasia type 2 as the tomograph can localize foveal pit enlargement. Which is a result of secondary loss of the outer nuclear layer and ellipsoid zone that can progress into large cysts (often called ‘cavitation’) that can encompass all retinal layers.
Automating the detection of macular telangiectasia type 2 with the help of AI image interpretation systems for OCT scan analysis is already possible thanks to Altris AI.
AI for Myelinated Retinal Nerve Fiber Layer
Myelinated nerve fiber layer (MRNF) is a disease that occurs in 1%. It is a benign clinical condition that results from an embryologic developmental anomaly whereby focal areas of the retinal nerve fiber layer fail to lose their myelin sheath.
OCT is an effective method of MRNF detection with the help of the detection of the RNFL layer. Such tools as Altris AI image interpretation models are even more accurate in retina layers detection and volume measurement thanks to their growing level of accuracy.
AI image interpretation for Myopia

Myopia is not an eye disease. It is an eye-focusing disorder that affects 25% of the world population at a younger age. There are 2 distinct types of myopia: pathological and non-pathological — each of the types has its symptoms and treatment prognosis. The visual function of the patients, as well as the high quality of life, can be preserved if myopia is detected early enough and treated appropriately. Myopia is often diagnosed by ophthalmologists and optometrists with the help of OCT, thanks to its fine cross-sectional imagery of retinal structures. Unlike biomicroscopy, angiography, or ultrasonography, OCT can reveal undetectable retinal changes in asymptomatic patients with myopia.
Current AI image interpretation models show great promise in detecting myopia on OCT scans, and their results can be compared to the results of junior retina specialists. Altris AI is an accurate AI tool for myopia.
AI for Pigment Epithelium Detachment
Retinal pigment epithelial detachment (PED) is often observed in Wet AMD and other conditions. It is determined as a separation of the RPE layer from the inner collagenous layer of Bruch’s membrane. With its capability to visualize retinal layers, OCT helps eye care specialists with timely PED diagnostics. Powered with AI image interpretation systems, OCT diagnostics can promise zero human errors and exceptional accuracy.
AI for Polypoidal Choroidal Vasculopathy (PCV)
Polypoid Choroidal Vasculopathy is a disease of the choroidal vasculature. Serosanguineous detachments of the pigmented epithelium and exudative changes that can commonly lead to subretinal fibrosis are the main OCT signs of PCV. AI image interpretation systems show great potential in establishing a difference in diagnostics between PCV and AMD.
AI for Preretinal Hemorrhage

Preretinal hemorrhage is a complication of many pathologies, such as leukemia or ocular/head trauma. Missing preretinal hemorrhage means putting a patient at risk. Preretinal hemorrhage can be a presenting sign of some systemic diseases. In any case, OCT diagnostics are performed to determine preretinal hemorrhage and its real reason.
AI image interpretation for Pseudohole
Sometimes the pulling or wrinkling of the epiretinal membrane (ERM) can result in a gap called a pseudohole. A pseudohole can look like a macular hole; sometimes, it can turn into one, so it is essential to distinguish between these two phenomena. Optical coherence tomography can accurately determine a pseudohole revealing an epiretinal membrane with contraction of the retina or suppression of retinal layers. Combined with AI image interpretation, OCT diagnosis can guarantee higher accuracy in pseudohole detection.
AI for Retinal Angiomatous Proliferation (RAP)
RAP is a subtype of AMD, which is neovascularization that starts at the retina and progresses posteriorly into subretinal space. There are 3 stages of RAP: intraretinal neovascularization (IRN), subretinal neovascularization (SRN), and choroidal neovascularization (CNV). OCT is effective for detecting IRN only since changes beneath the pigment epithelium are challenging to assess. AI image interpretation models effectively differentiate between RAP and polypoidal choroidal vasculopathy (PCV), comparable to the performance of eight ophthalmologists. AI cannot substitute eye care specialists but can be an excellent decision-making support tool.
AI for Retinal Detachment
Retinal detachment is a serious eye condition that happens when the retina pulls away from the tissue around it. It can be a result of trauma or another disease. OCT has become a new standard for detecting early retinal detachment and defining the best time for surgical operation, for example. OCT powered with AI image interpretation systems can give eye care specialists the confidence they need to determine the degree of detachment and make the correct prognosis.
AI for Retinitis Pigmentosa
RP is a hereditary diffuse pigment retinal dystrophy characterized by the absence of inflammation, progressive field loss, and abnormal ERG. OCT diagnostics allows assessing morphological abnormalities in RP, providing insights into the pathology of RP and helping to make a good prognosis. AI image interpretation applied for the OCT analysis shows promising results in Inherited Retinal Diseases detection and future management.
AI image interpretation for Retinoschisis

Retinoschisis is an eye condition characterized by a peripheral splitting of retinal layers. OCT is an effective method of retinoschisis diagnostic. The application of AI image interpretation tools for OCT analysis for identifying retinoschisis (among other myopia conditions) is comparable to the performance of experienced ophthalmologists.
AI for Retinal Pigment Epithelial (RPE) Tears (Rupture)
RPE rupture or RPE tears is the condition when this retinal layer acutely tears from itself and retracts in an area of a retina, usually overlying a pigment epithelial detachment (PED). OCT is an effective method of diagnostics of RPE tears. OCT scans will show a discontinuity of the hyperreflective RPE band, with a free edge of RPE usually wavy and scrolled up overlying the PED, contracted back towards the CNVM.
AI image interpretation systems can provide eye care specialists with confidence when detecting RPE tears. Systems such as Altris AI can distinguish between retinal layers with exceptional accuracy, exceeding the accuracy of eye care specialists.
AI for Solar Retinopathy (Maculopathy)
Solar retinopathy is photochemical toxicity and the consequent injury to retinal tissues located in the fovea in most cases. OCT helps to diagnose solar retinopathy by indicating changes and focal disruption at the level of the subfoveal RPE and outer retinal bands. The overall retinal architecture remains intact. AI image interpretation models can confidently assist eye care specialists in detecting solar retinopathy, even when they are in doubt.
AI for Subhyaloid Hemorrhage
Subhyaloid hemorrhage is diagnosed when the vitreous is detached from the retina because of blood accumulation. This type of hemorrhage is rare and is different from intraretinal hemorrhage caused by trauma or diabetes. OCT helps to detect subhyaloid hemorrhage. For eye care specialists who don’t have experience in detecting subhyaloid hemorrhage, the AI image interpretation model can become a great support tool.
AI for Subretinal Fibrosis
Subretinal fibrosis appears due to wound healing reaction to the choroidal neovascularization in nAMD or other conditions. Early diagnostics of subretinal fibrosis are critical because a neovascular lesion’s transformation into a fibrotic lesion can be very rapid. OCT is regarded as the most accurate method of diagnostics today.
AI image interpretation systems can help eye care specialists who use OCT with early diagnostics of subretinal fibrosis and improve patient outcomes.
AI for Subretinal Hemorrhage
Subretinal hemorrhages are a complication of various diseases which arise from the choroidal or retinal circulation. It is most often caused by AMD, trauma, and retinal arterial macroaneurysm. OCT will be an effective tool for determining the level at which subretinal hemorrhage occurred. Powered with AI image interpretation models, OCT can become the decision-making support tool eye care specialists need for subretinal hemorrhage identification.
AI for Sub-RPE (Retinal Pigment Epithelial) Hemorrhage
Sub-RPE (retinal pigment epithelium) hemorrhage is located between the RPE and Bruch’s membrane. OCT is an essential tool for validating the hemorrhage’s diagnosis and localization. AI image interpretation tools, such as Altris AI, will ensure that Sub-RPE hemorrhage is not missed.
AI for Tapetoretinal degeneration or dystrophy
Tapetoretinal dystrophy or tapetoretinal degeneration (TD) is exogenous destruction of the retina caused by a genetic mutation. Eye care specialists might easily miss such rare conditions as tapetoretinal degeneration. It is often advisable to have AI image interpretation systems as a decision-making support tool not to miss TD or other uncommon diseases.
AI image interpretation for Vitelliform Dystrophy
It is autosomal dominant degenerative maculopathy wherein a mutation in the bestrophin gene leads to lipofuscin accumulation in RPE cells manifested in a yellow spot. Detecting vitelliform dystrophy is critical at the early stages as it can lead to vision loss. OCT provides essential information on the lesion’s morphology, location, and dynamics. Empowered with AI image interpretation tools, such as Altris AI, eye care specialists won’t miss such a rare disease as vitelliform dystrophy at the early stage.
AI for Vitreomacular Traction Syndrome
Vitreomacular traction syndrome is a pathological condition characterized by a posterior vitreous detachment that leads to blurred vision or serious vision impairment. OCT is an essential method of diagnostics of vitreomacular traction syndrome as it can show the amount of involvement and tension on the macula caused by VMT. Combined with AI image interpretation tools, OCT analysis can give incredible results.
AI image interpretation for Wet AMD

Wet AMD is the most widespread disease among the elderly population in developing countries. It is a disease characterized by abnormal blood vessel growth under the retina. Understanding that this disease can lead to rapid and severe vision loss, its early detection and treatment are very important. OCT is a golden standard for the diagnostics of wet AMD as it shows fluid or blood underneath the retina without dye, among other pathological signs.
Today AI shows promising results in predicting the development of wet AMD based on OCT images. For instance, the DARC algorithm designed for detecting apoptosing retinal cells could predict new wet-AMD activity. Another effective AI image interpretation algorithm determines the location and volumetric information of macular fluid within different tissue compartments in wet AMD, providing eye care specialists with the ability to predict visual acuity changes.
AI for X-linked Juvenile Retinoschisis (XLRS)
XLRS is a rare congenital retina disease caused by mutations in the RS1 gene, which encodes retinoschisin, a protein involved in intercellular adhesion and likely retinal cellular organization. The disease usually affects younger males in their teenage years who complain about blurred vision. OCT is used to detect schisis in the superficial neural retina and thinning of the retina. Despite the lack of research articles on AI in OCT diagnostics of XLRS, there are AI image interpretation tools that already cope with this task effectively.
Final Words
Artificial intelligence can identify, localize, and quantify pathological signs in almost every disease of the macula and retina. That is how AI image interpretation systems can provide decision-making support with the pathologies at their early stages or rare pathologies. AI can help to detect many pathologies that are invisible to the human eye because of their size or that are at their early stage.
The overall potential of artificial intelligence for ophthalmologists and optometrists is enormous and includes pathological scan selection and scan analysis with the probability of existing pathologies and pathological signs. One trial is worth a thousand words in the case of AI tools for ophthalmologists and optometrists.

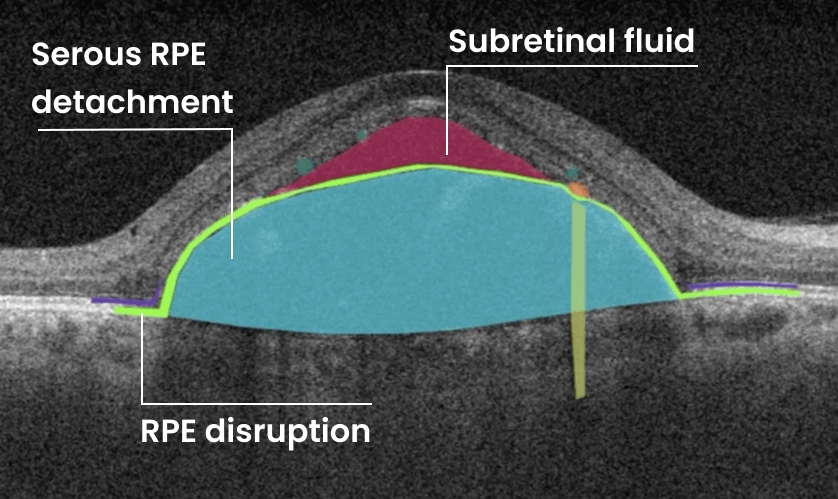

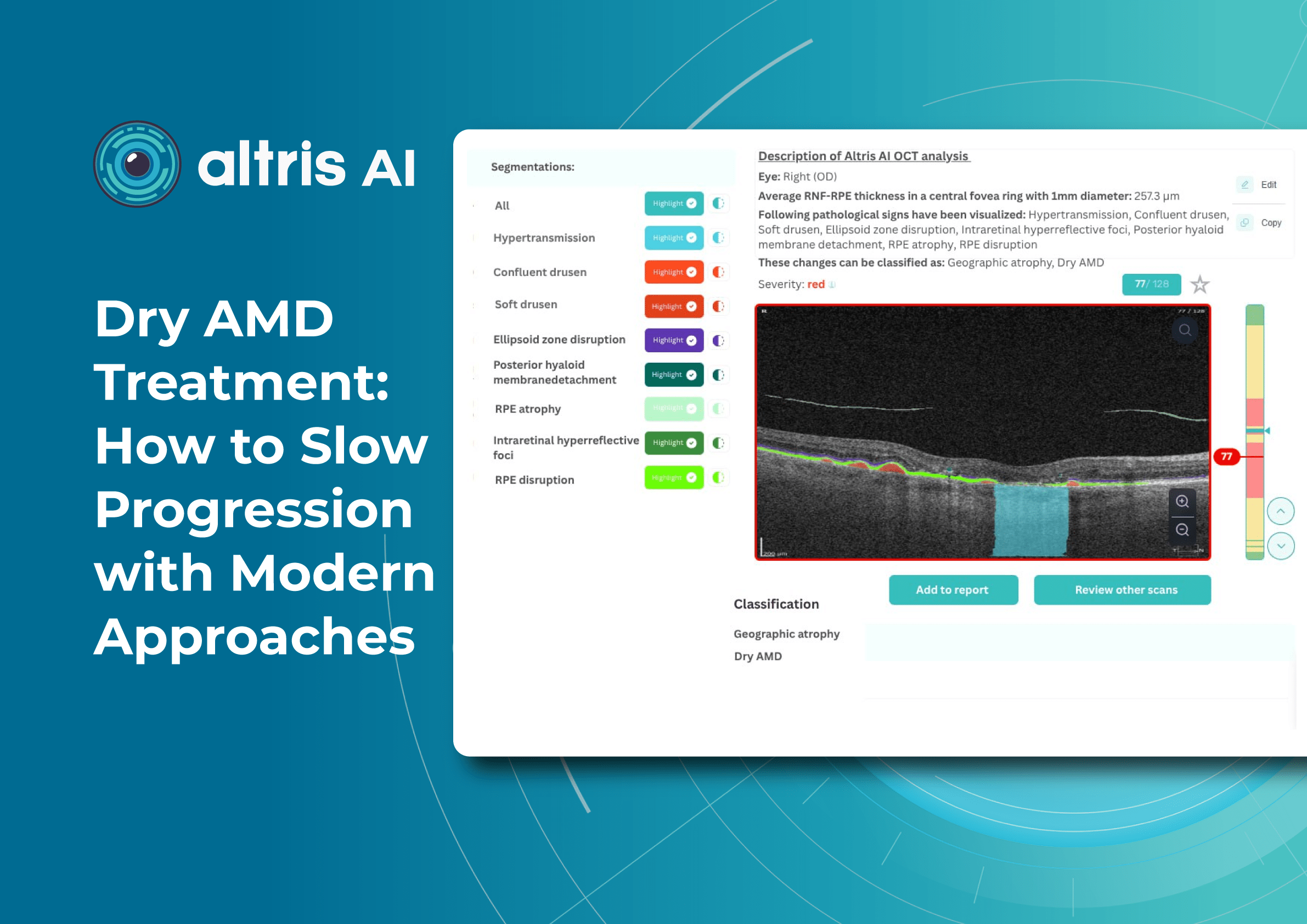

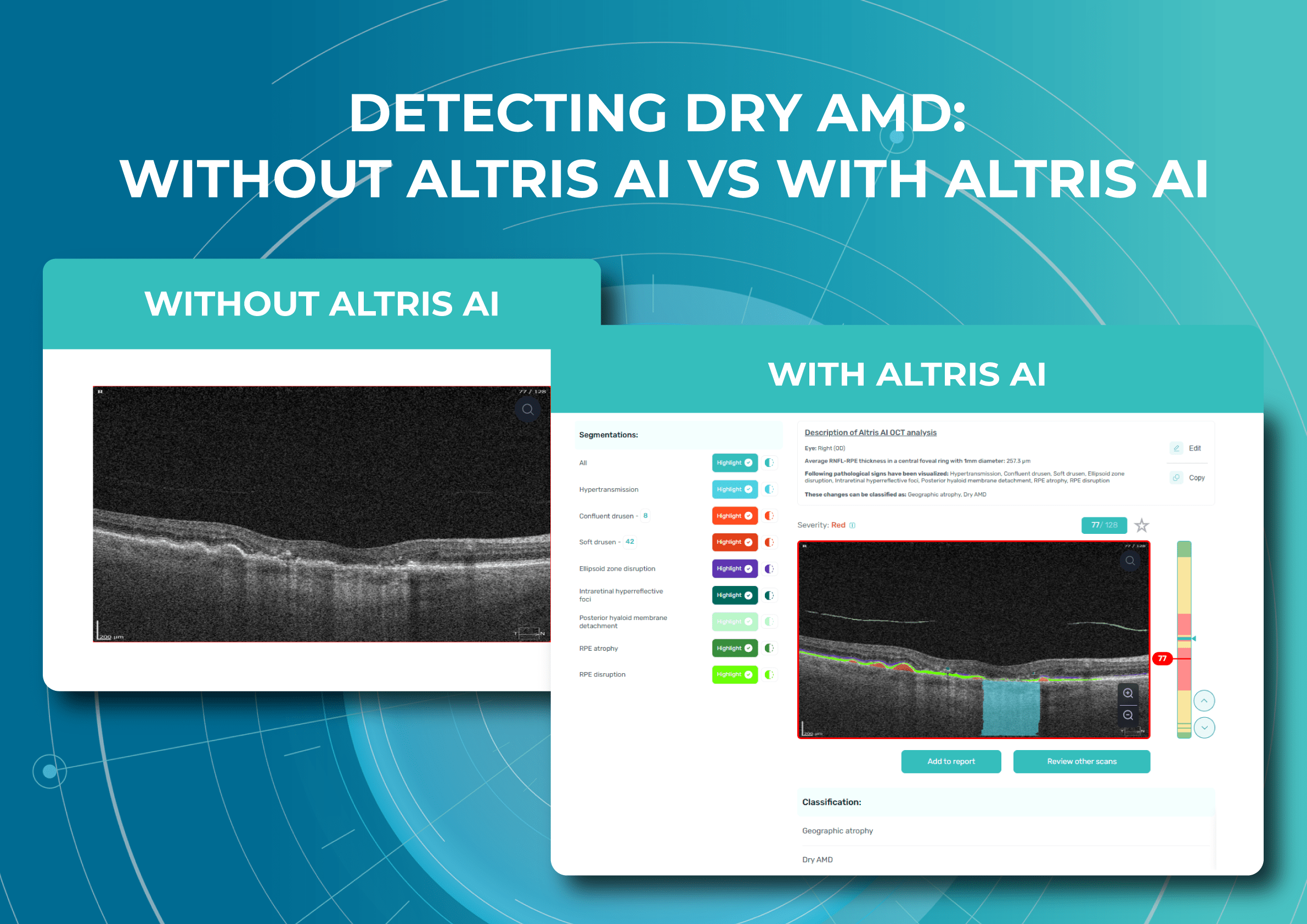
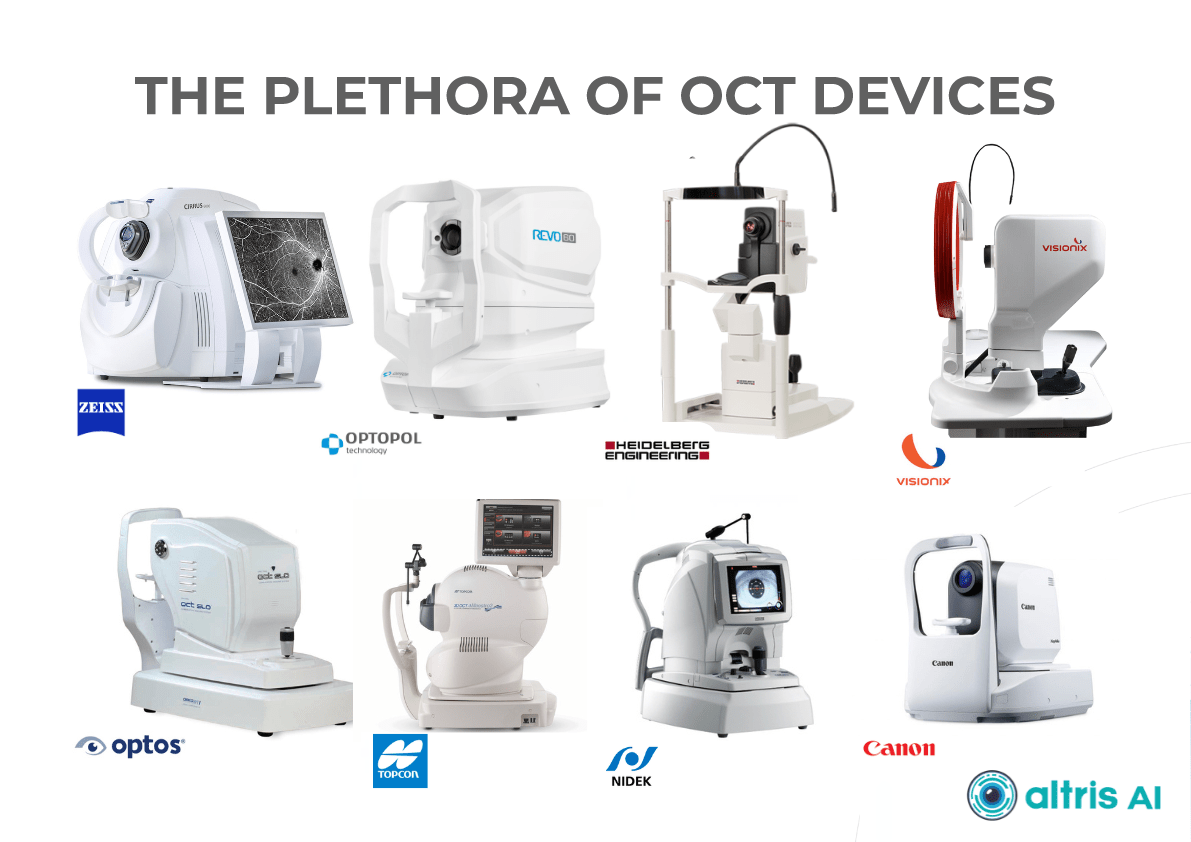
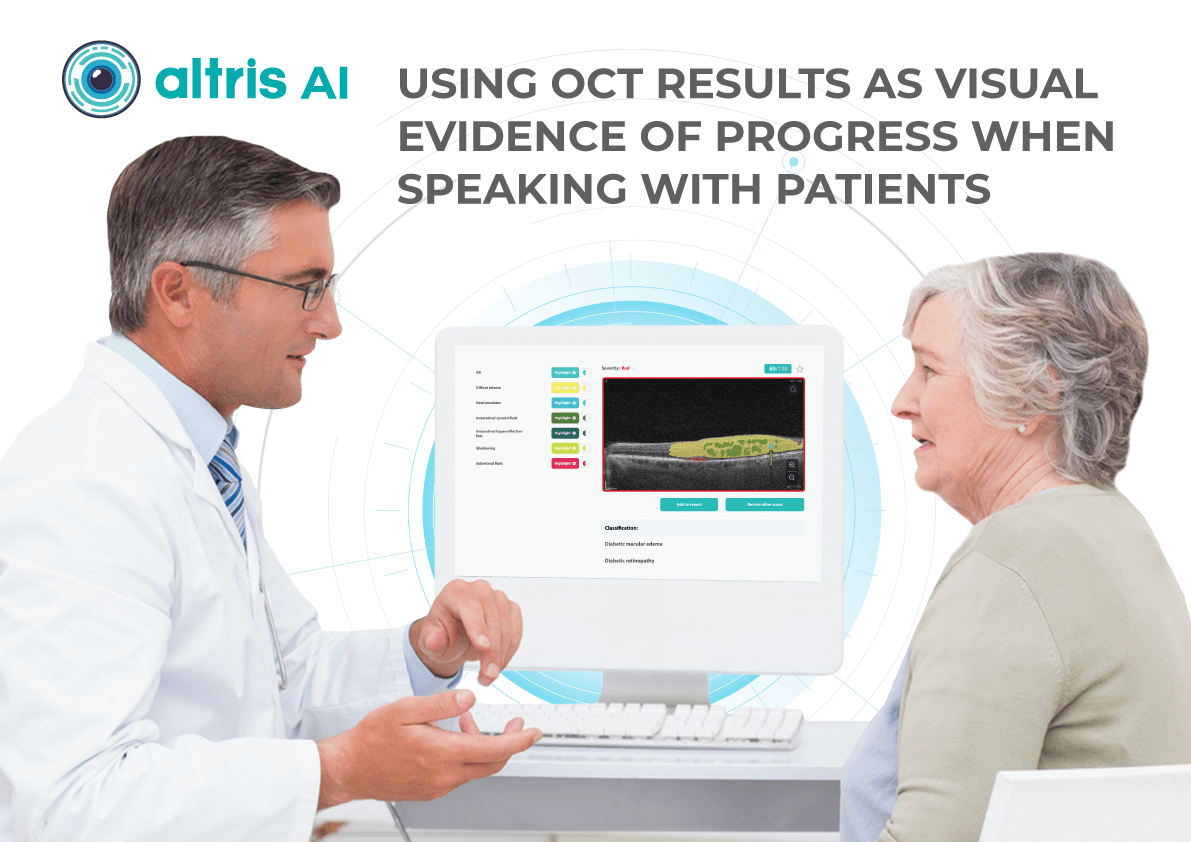








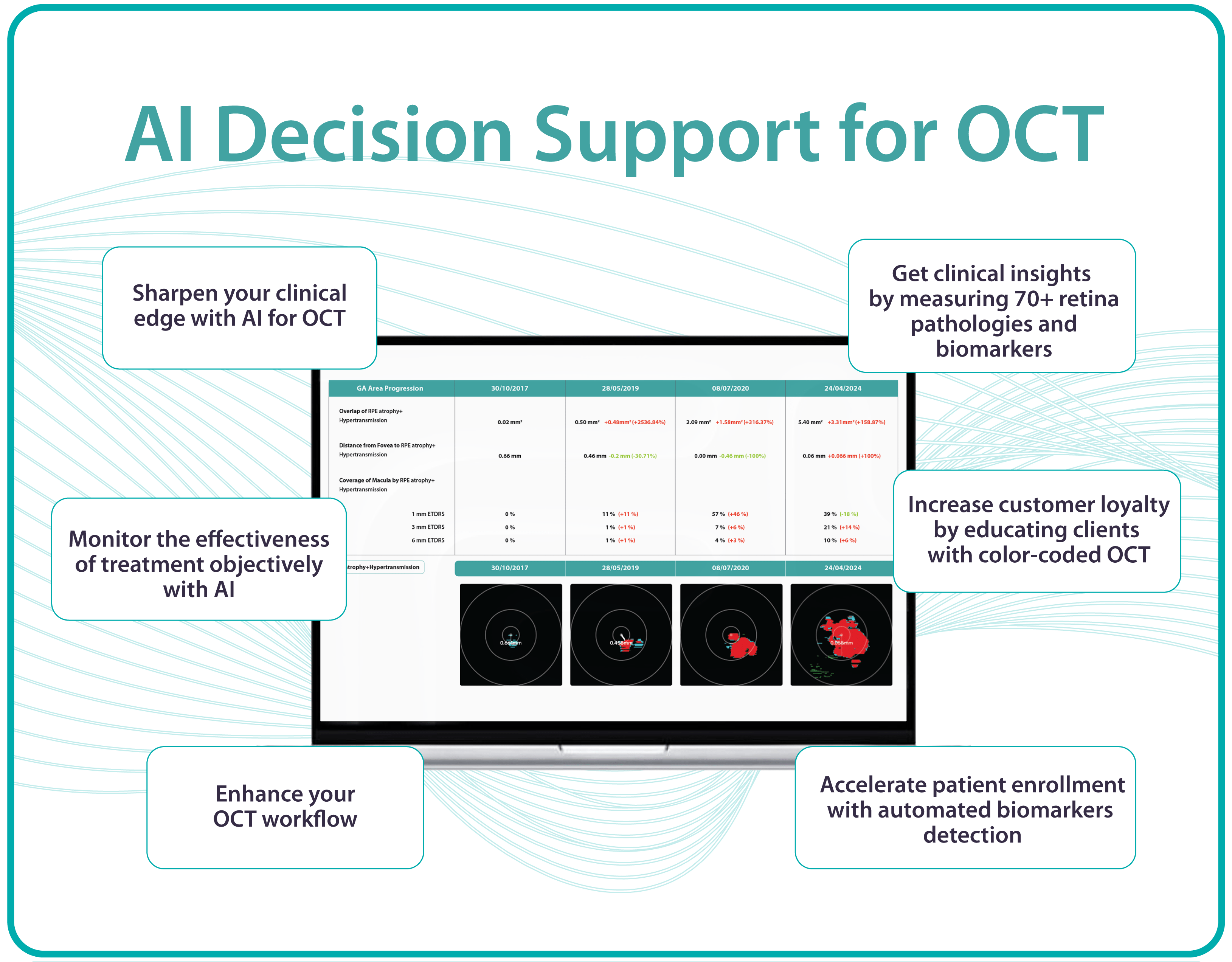

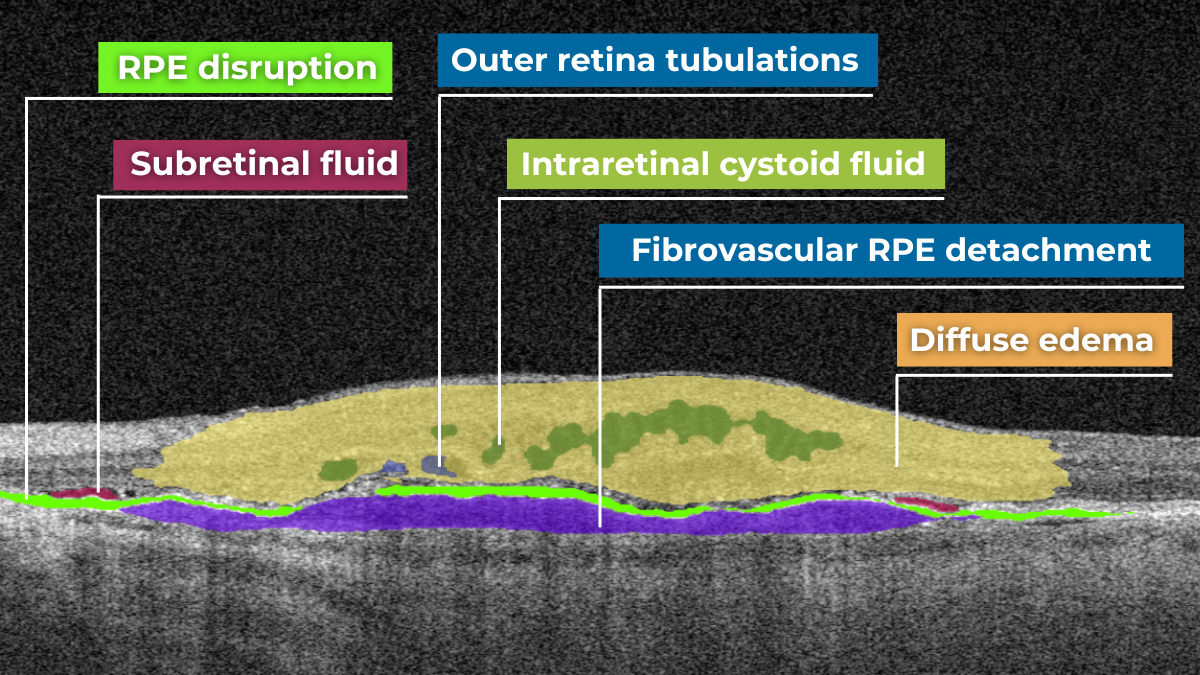
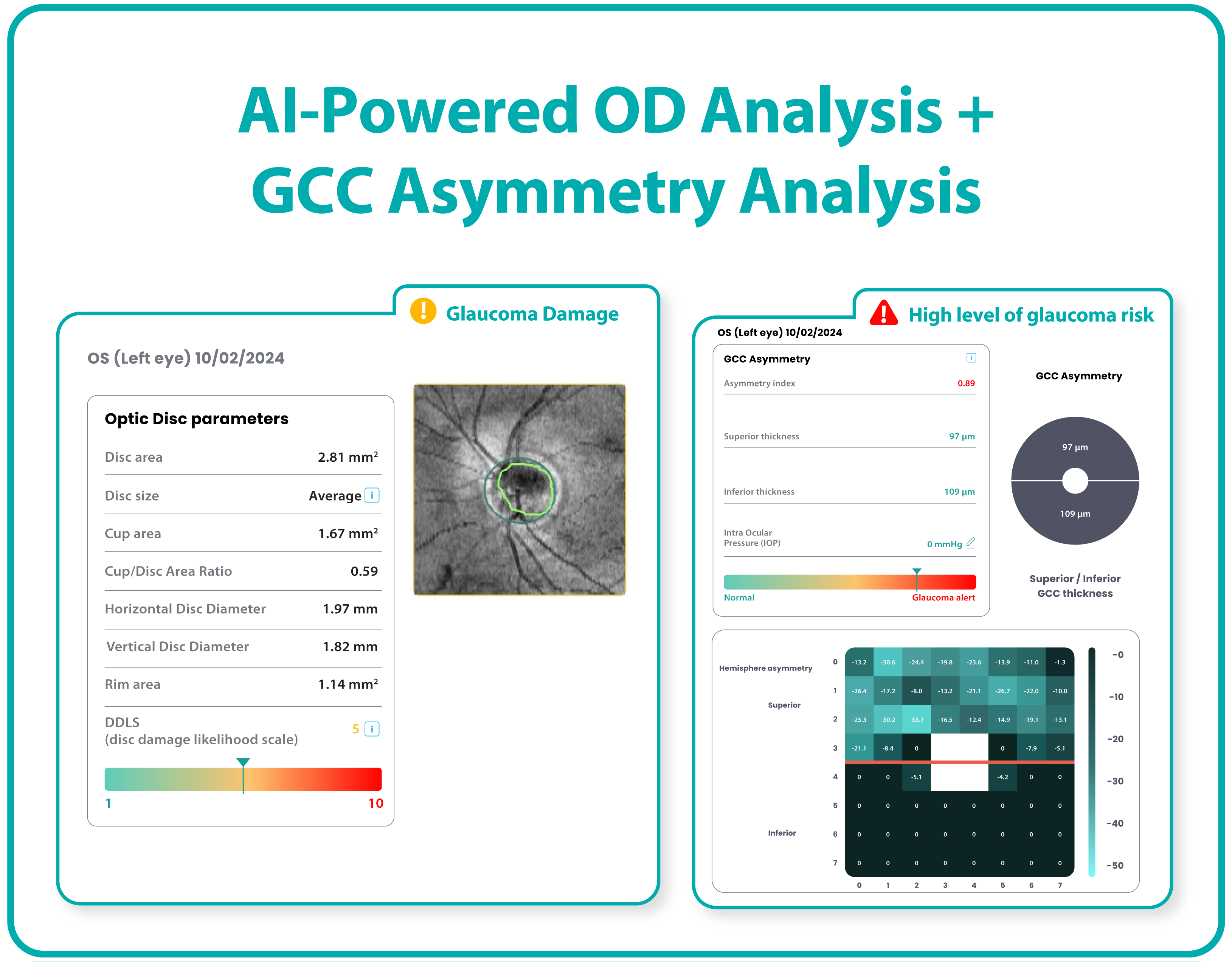



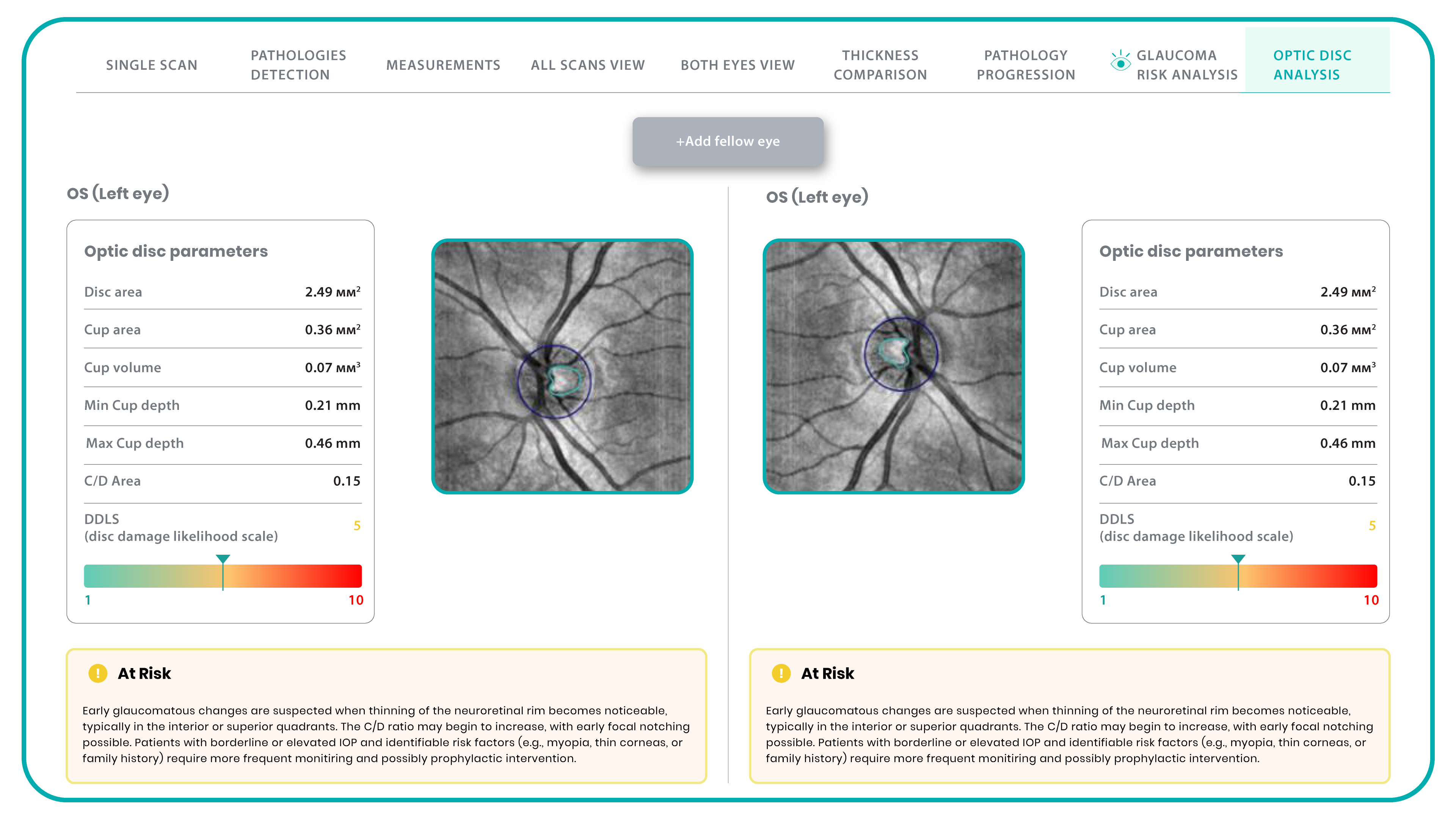









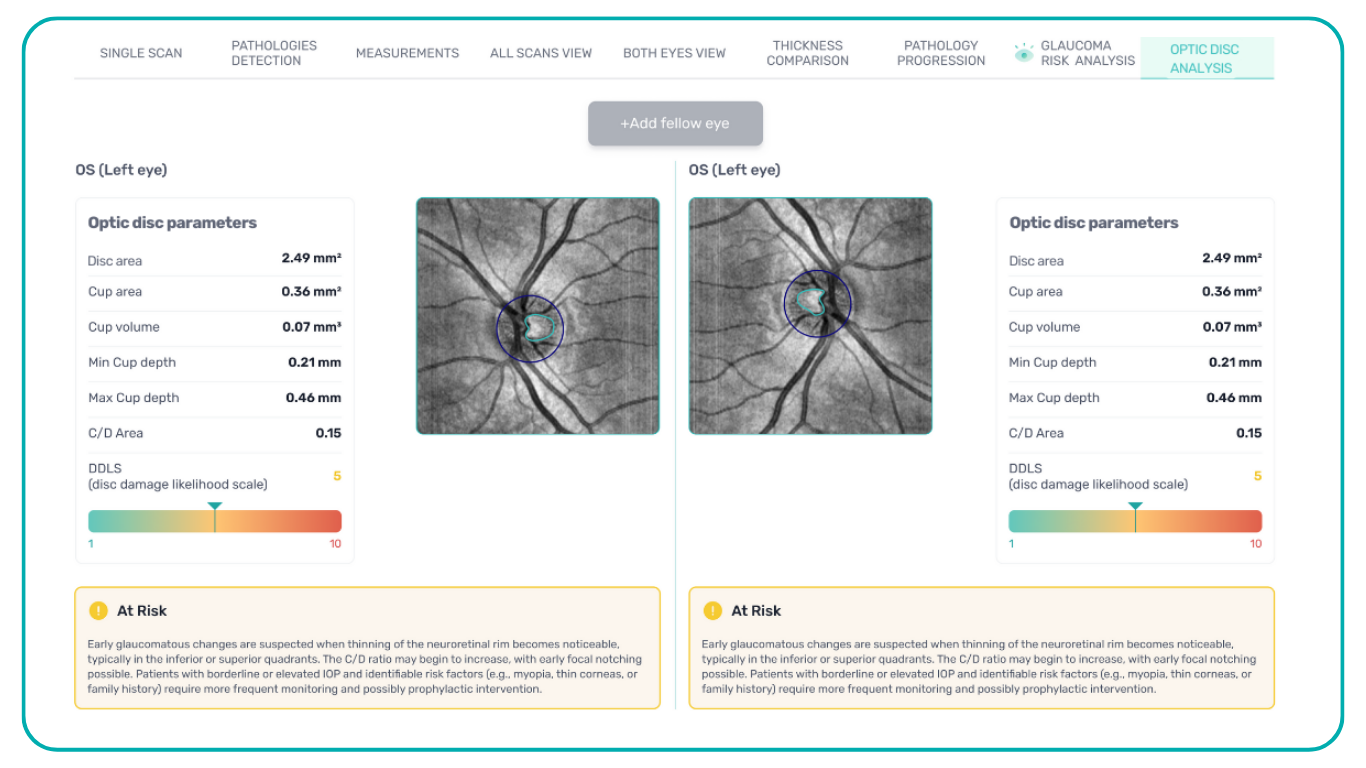
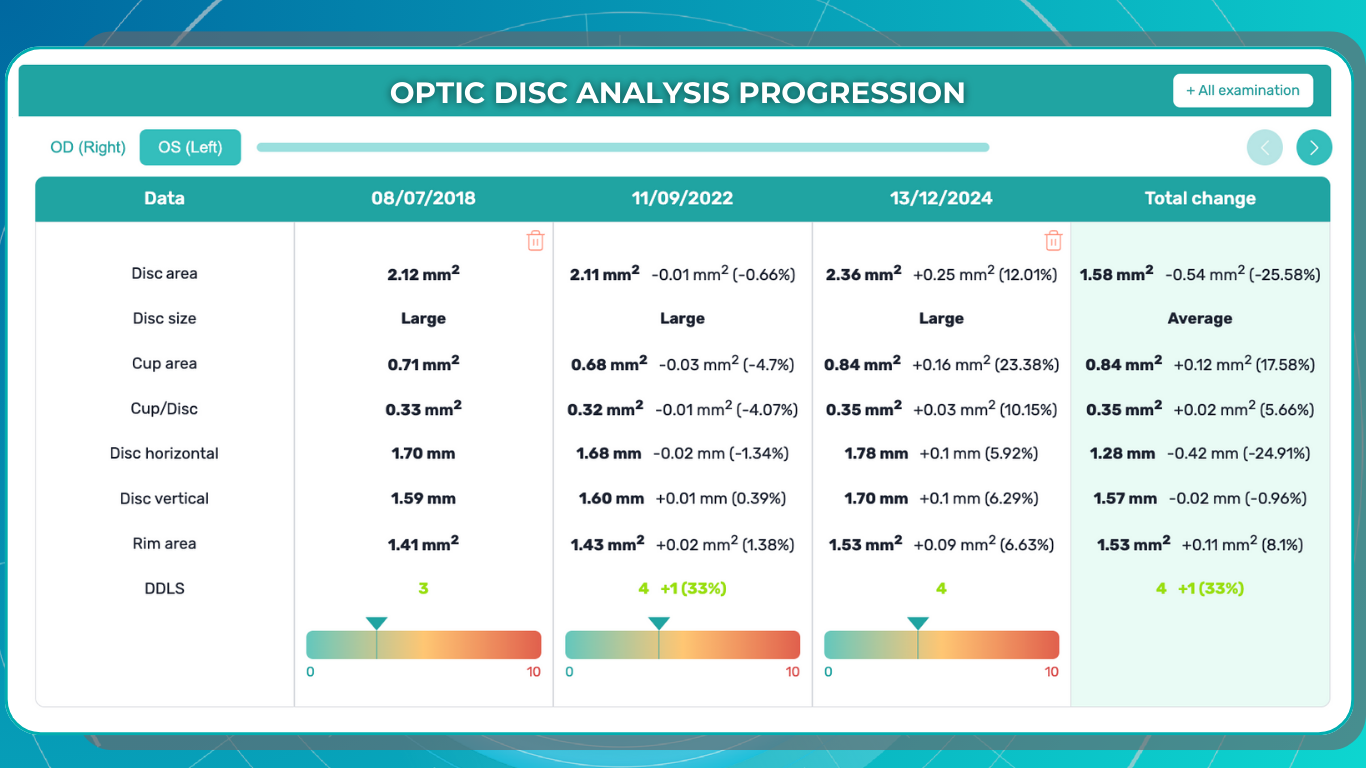

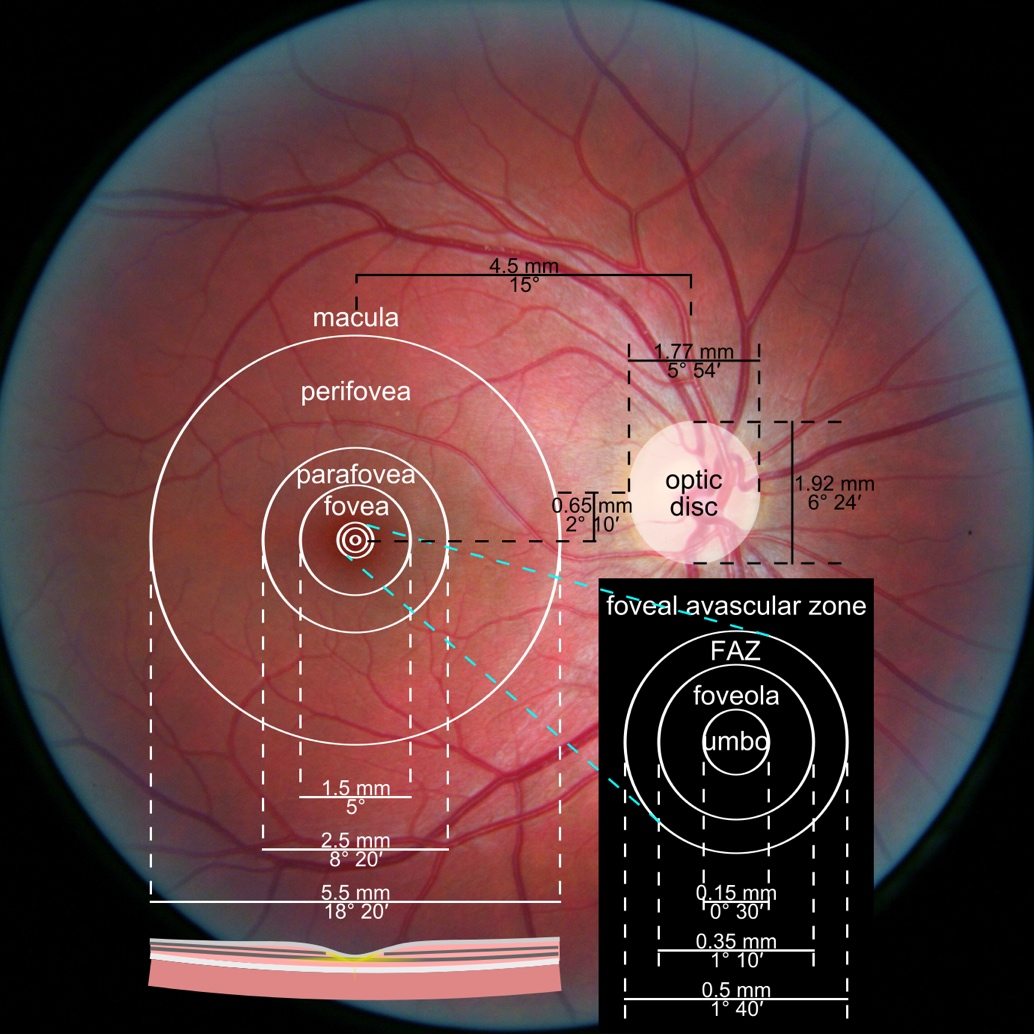
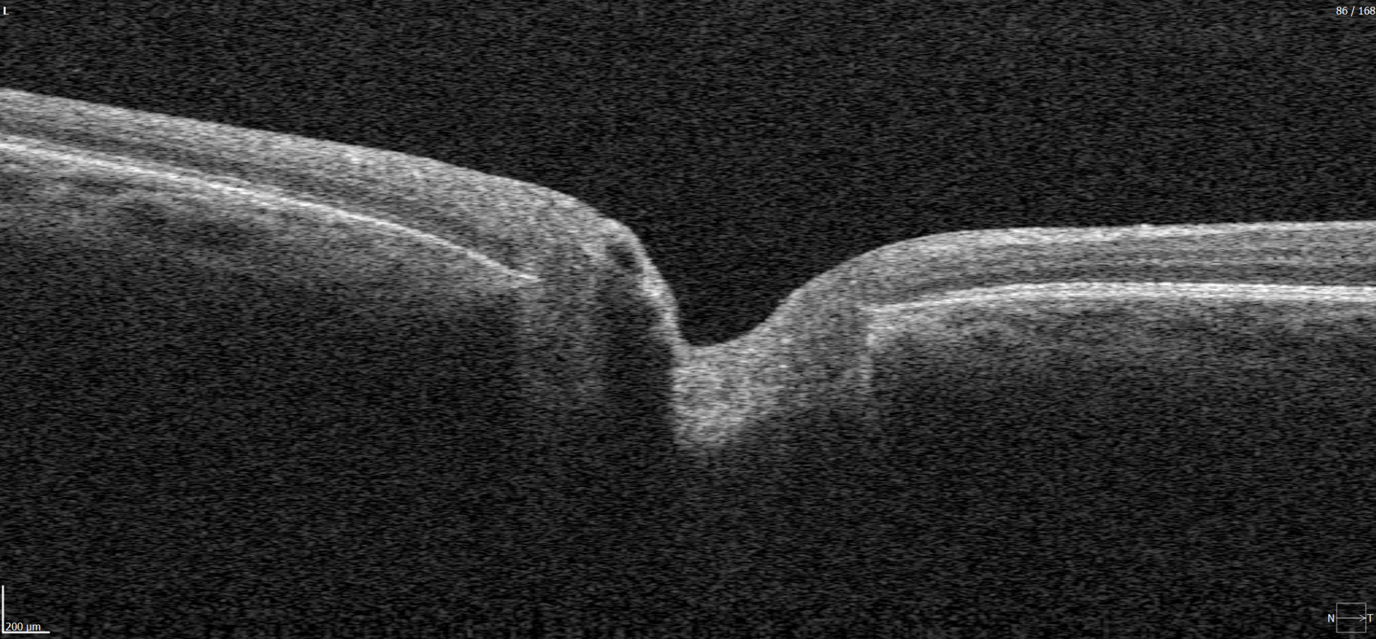
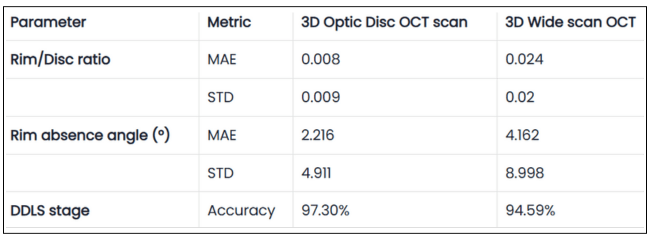
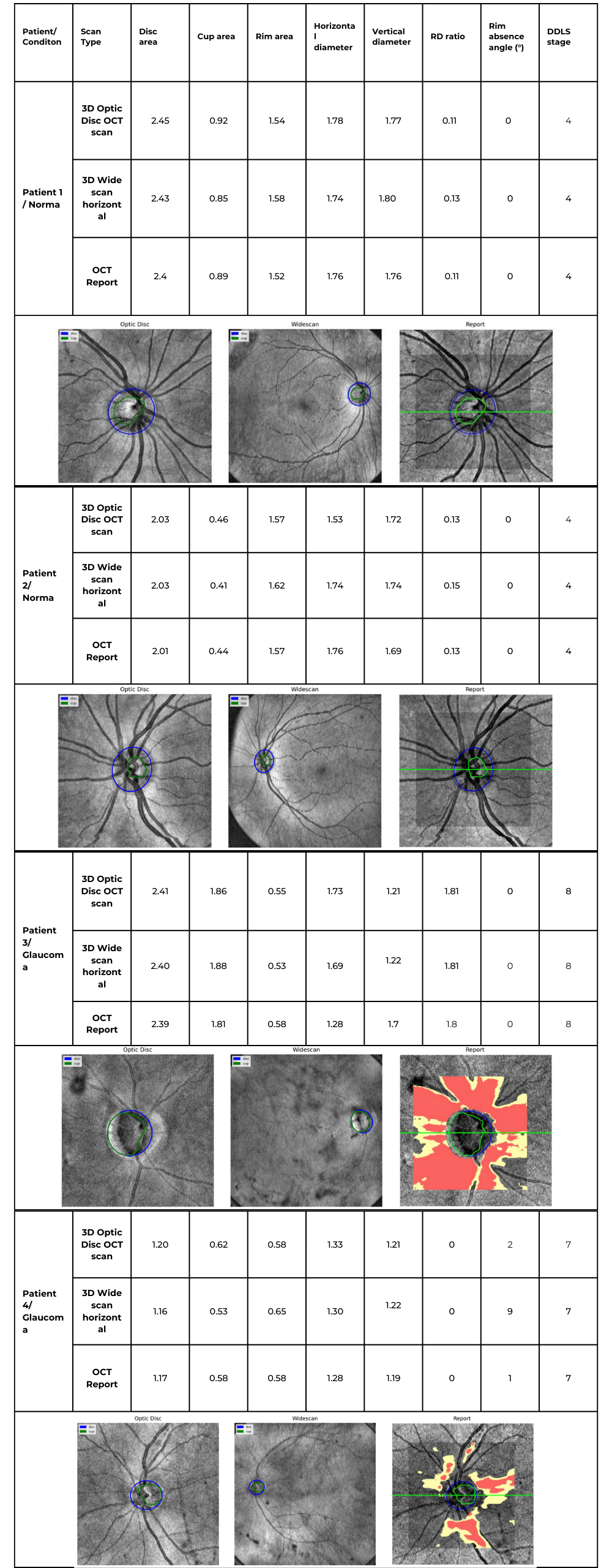
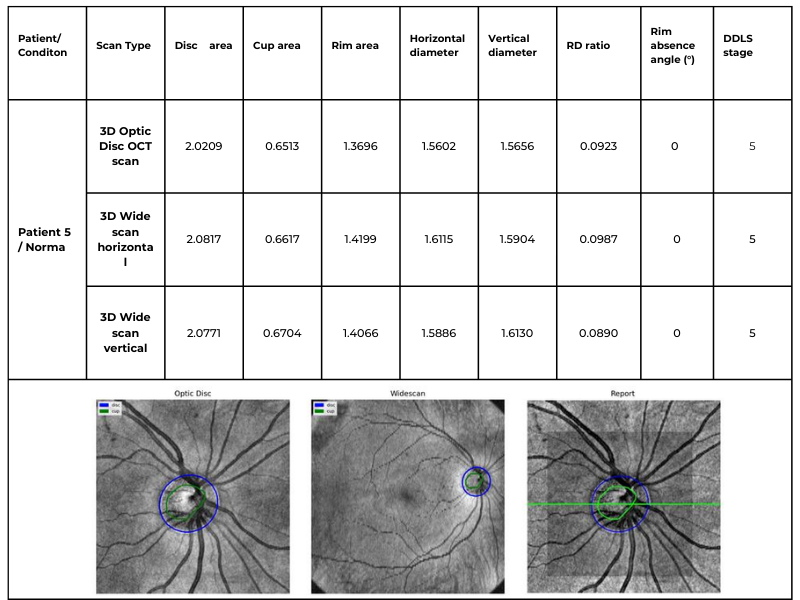
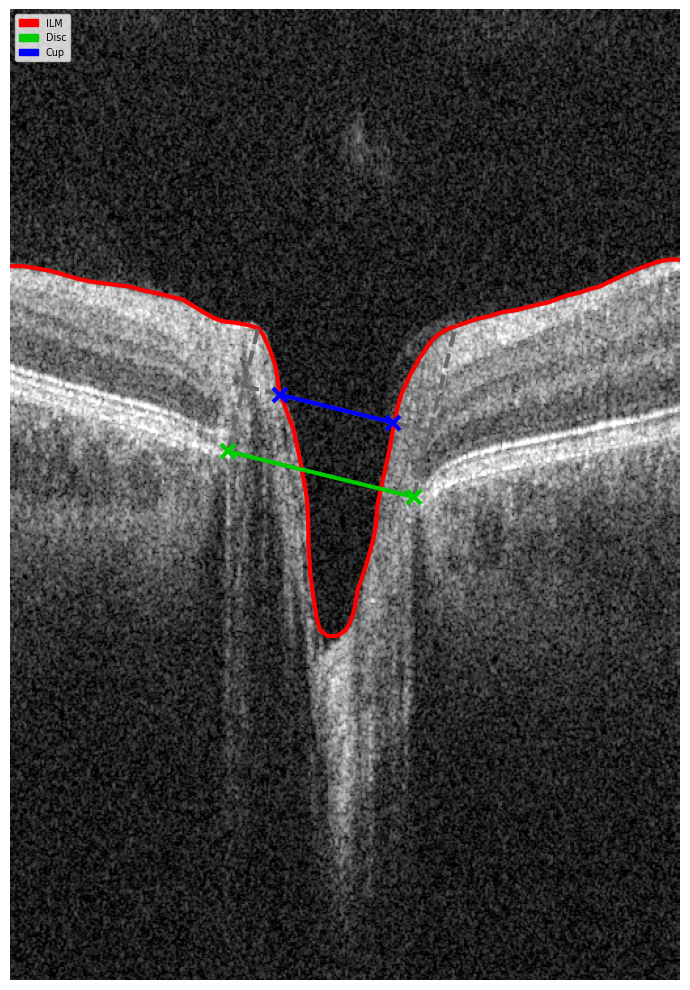
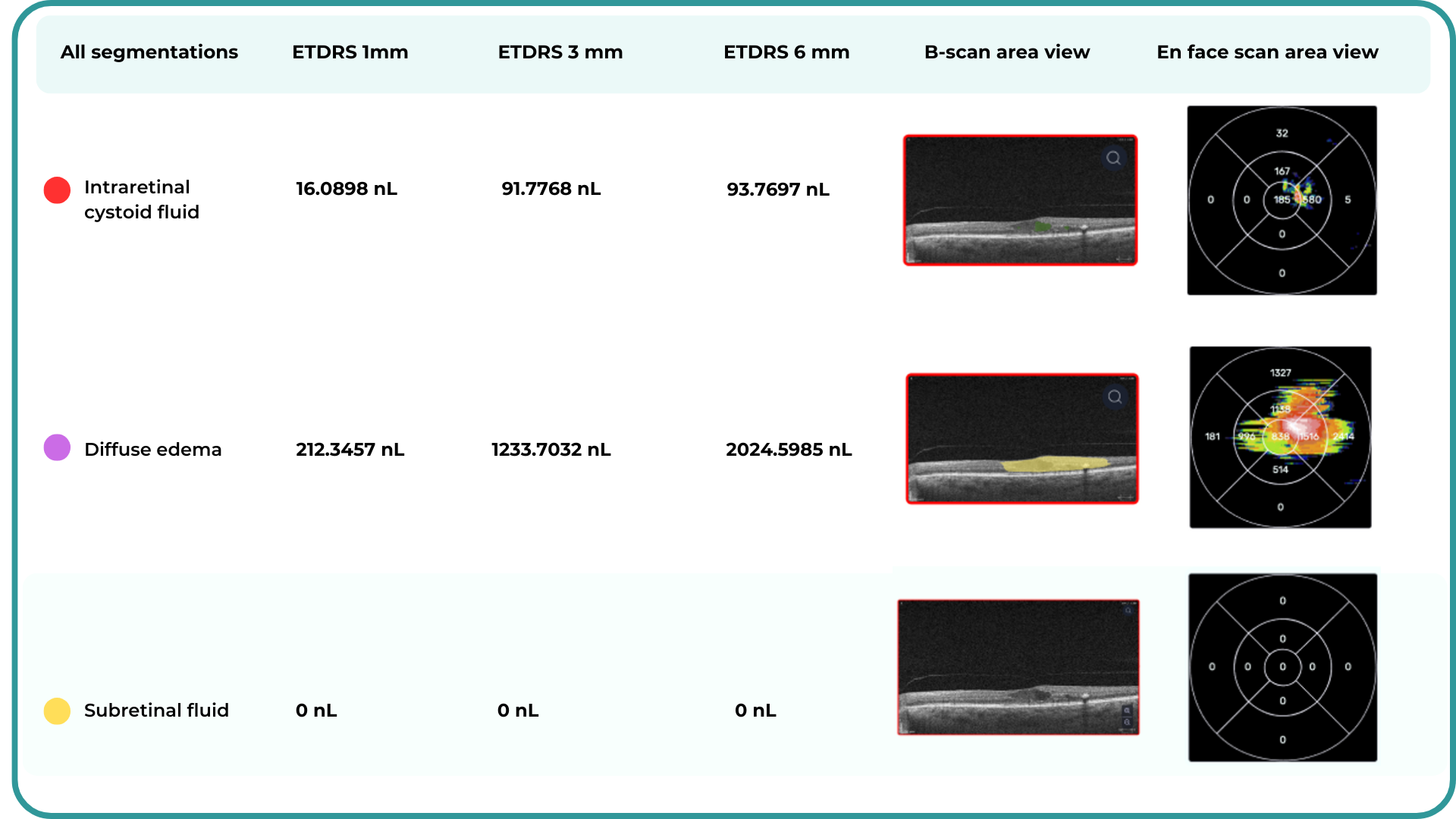
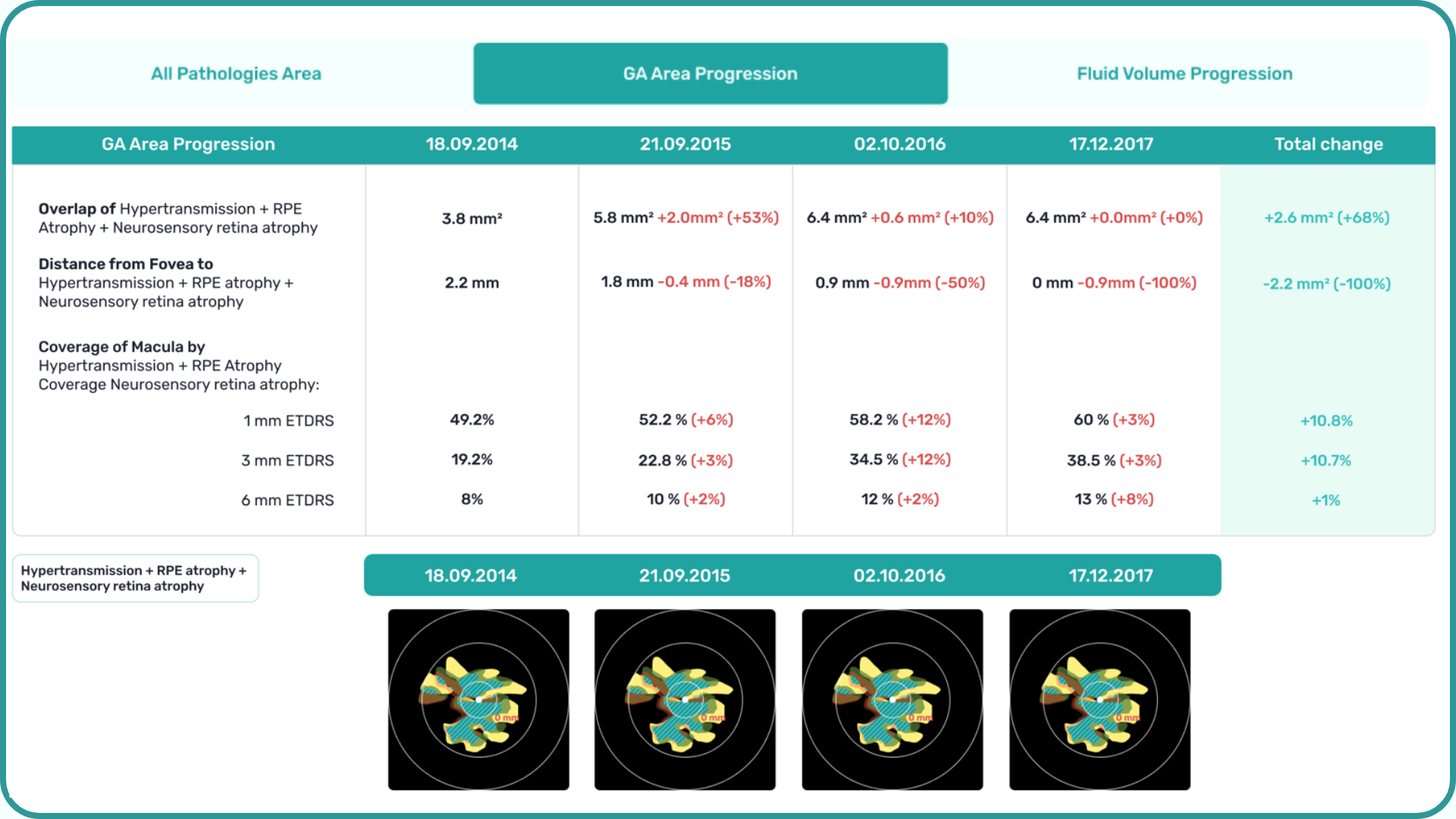
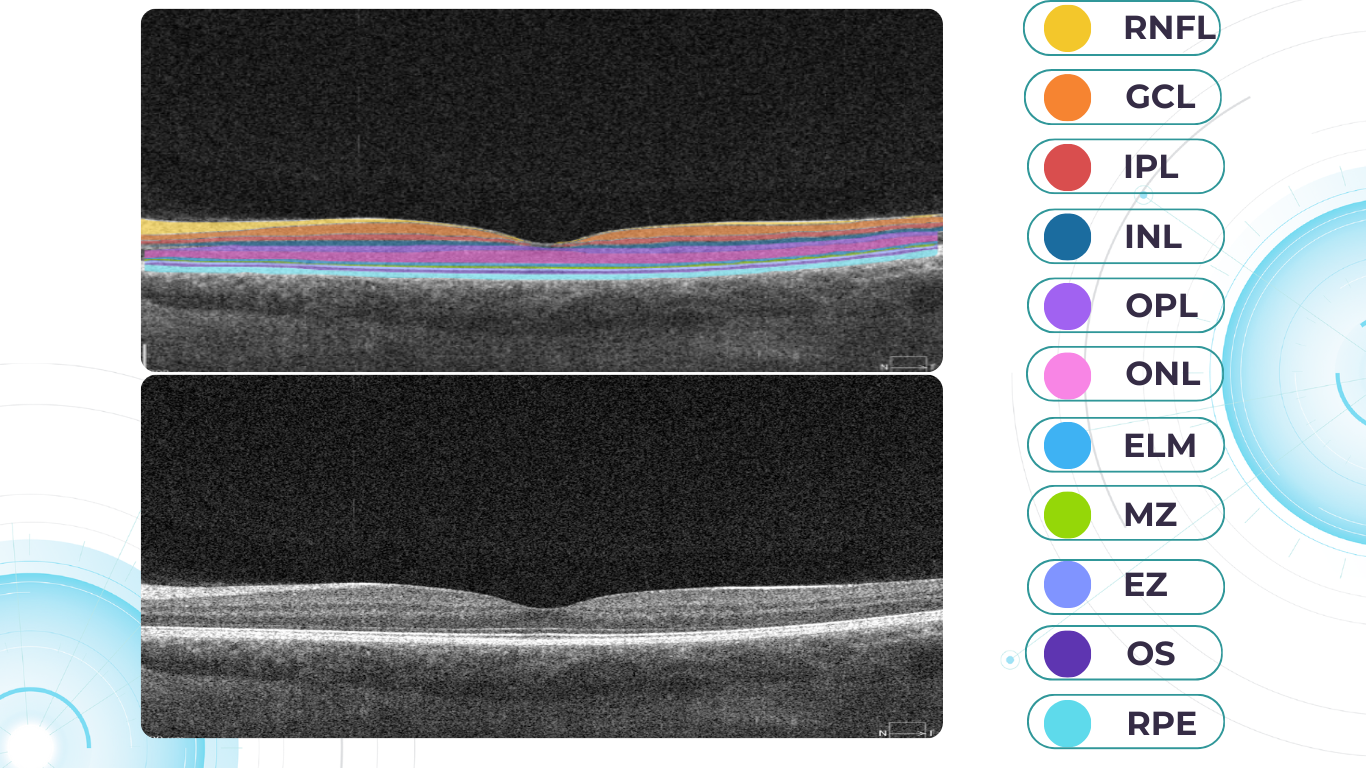
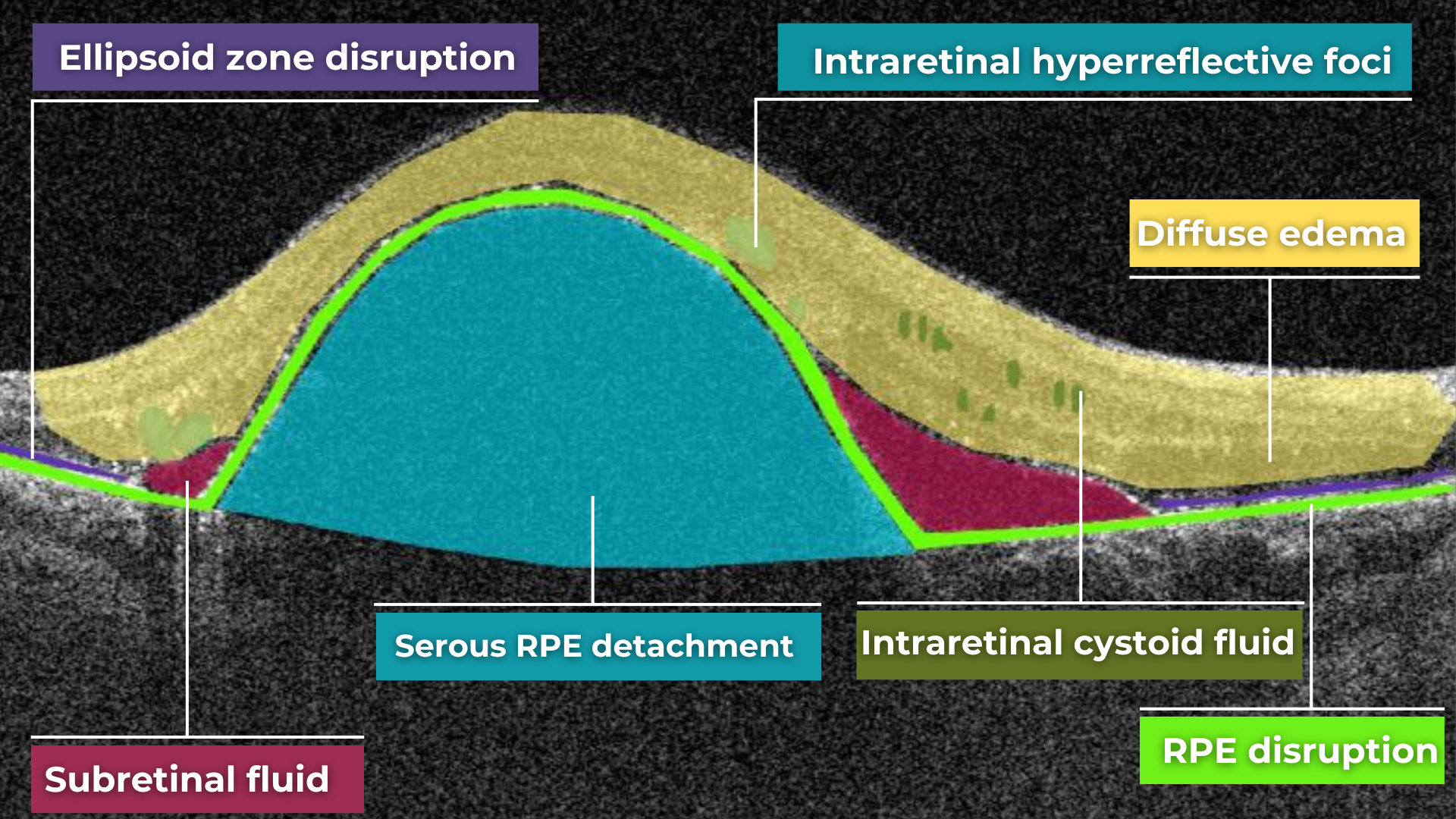
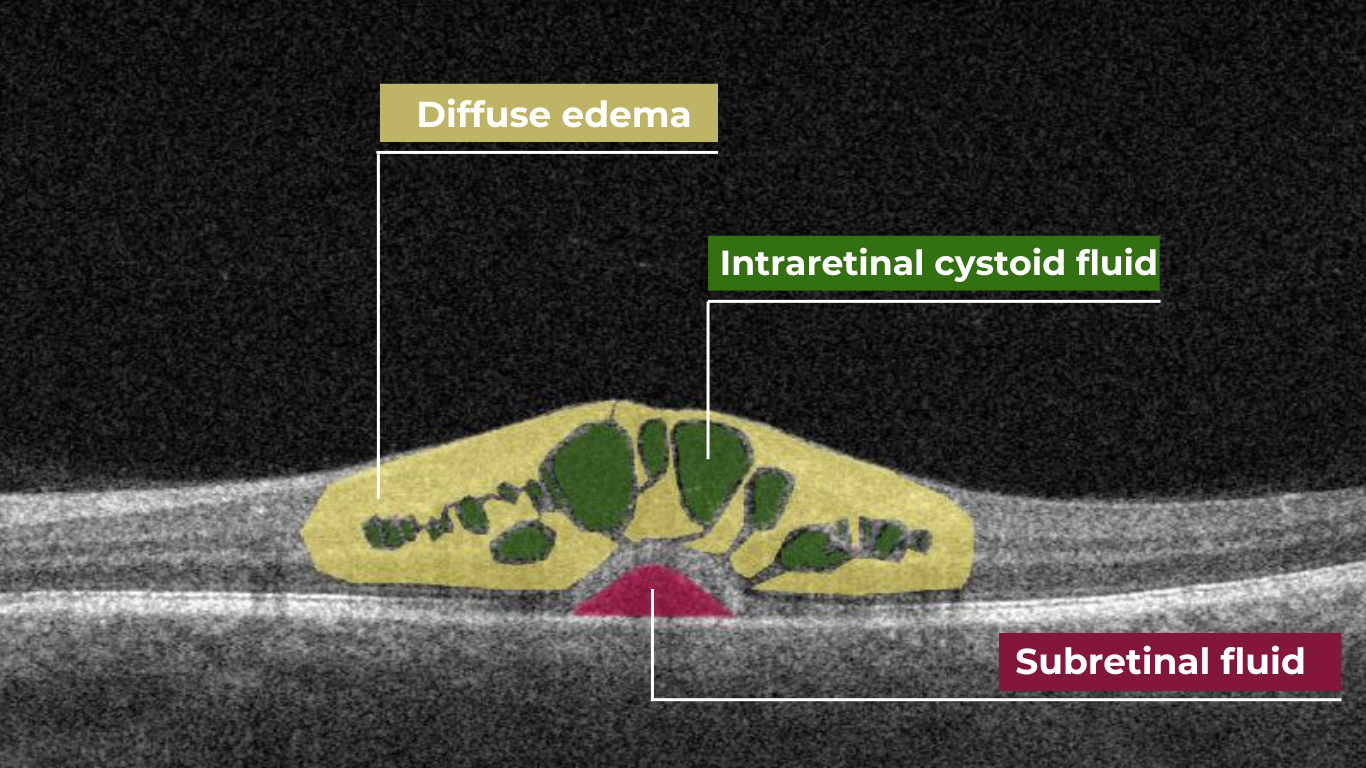
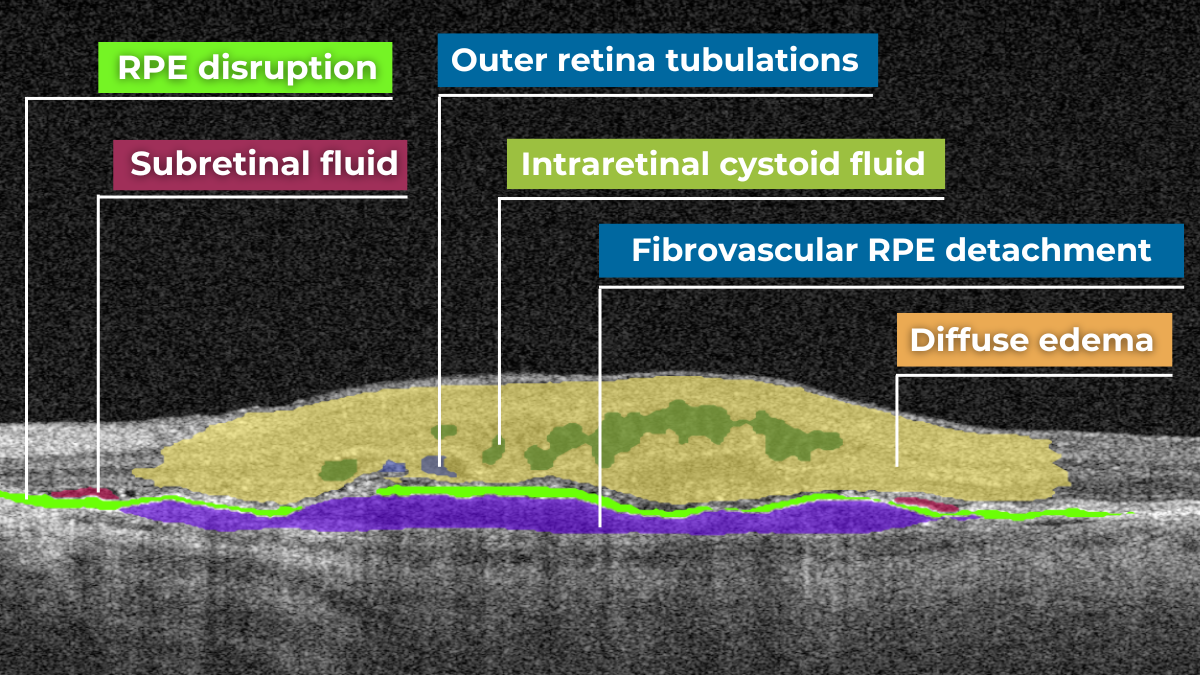
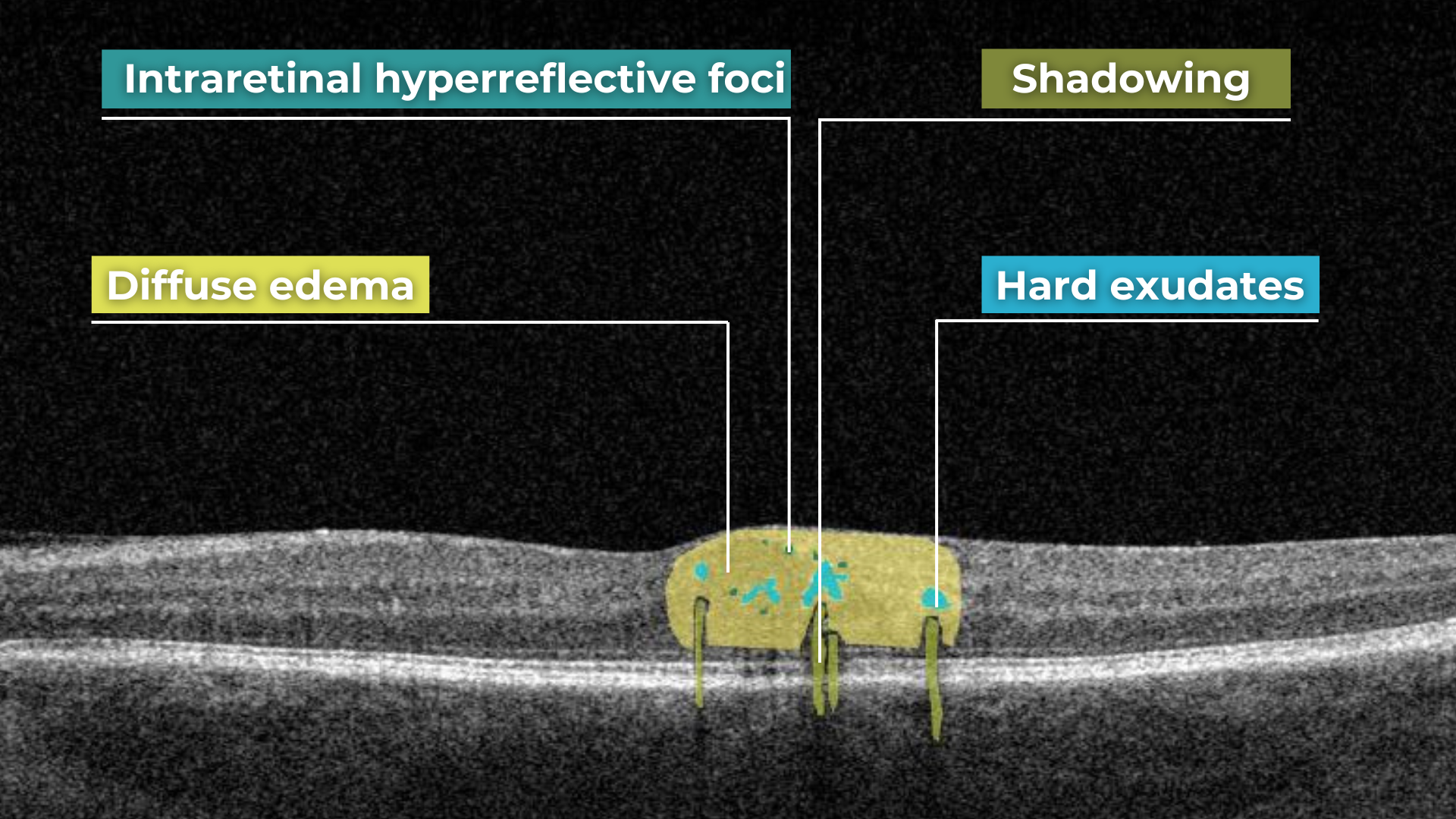
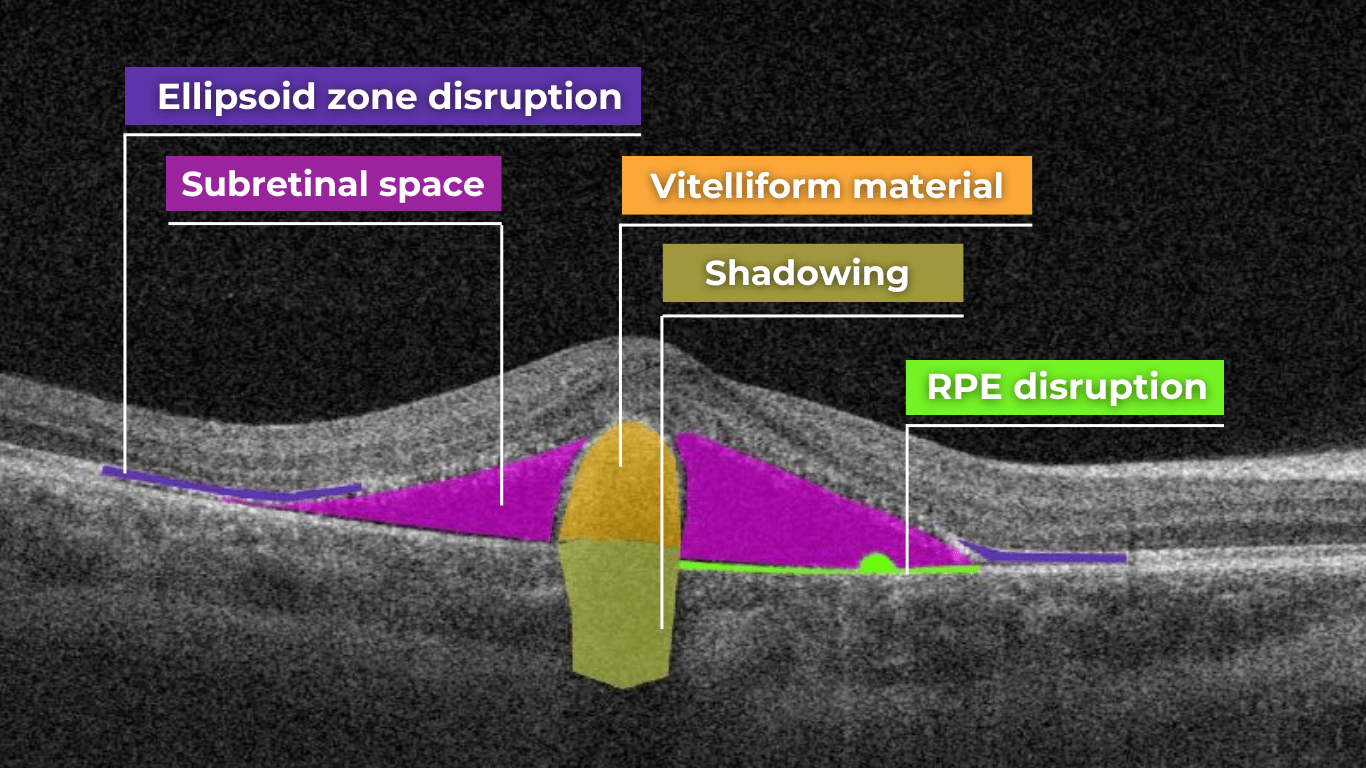
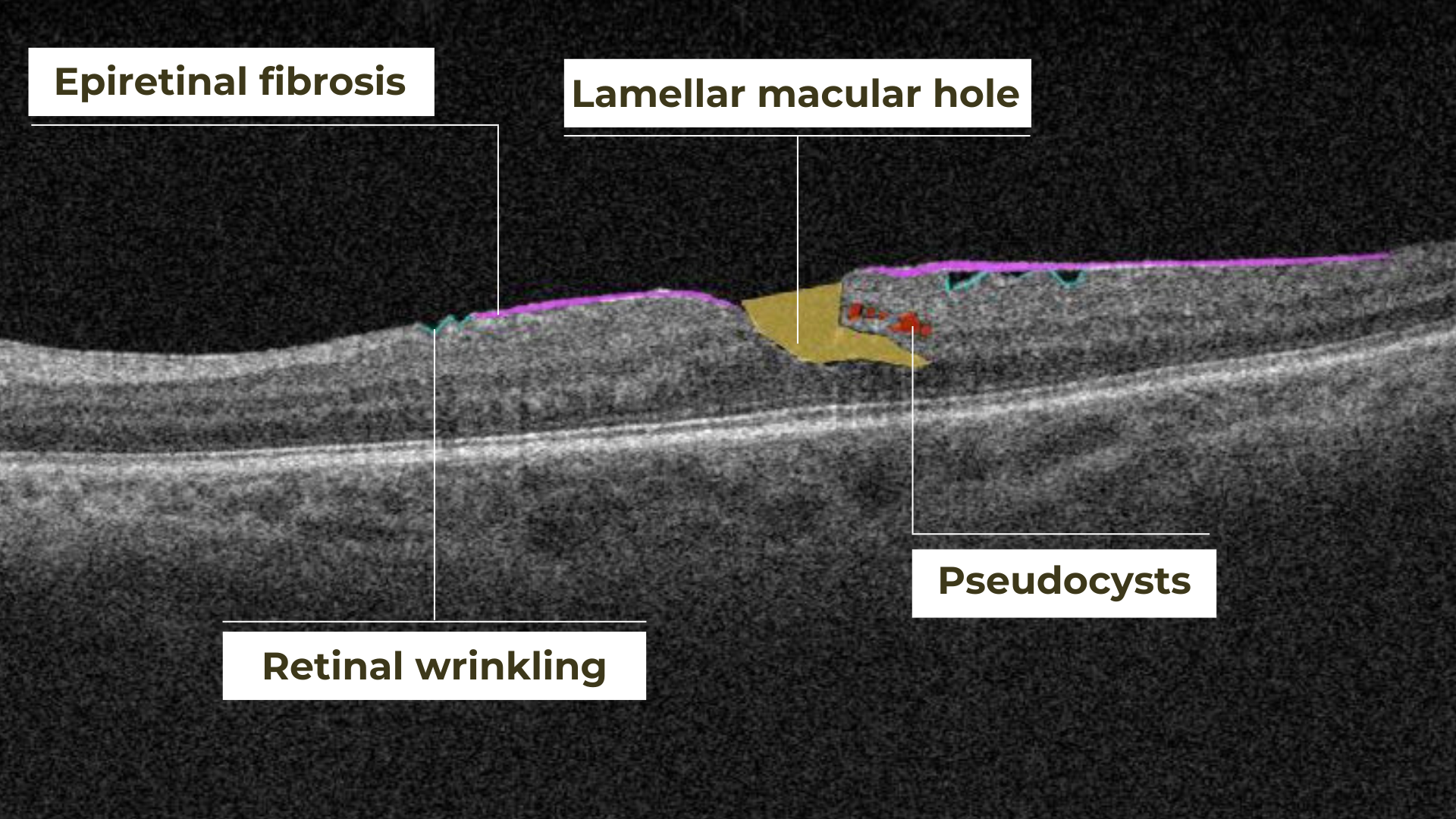
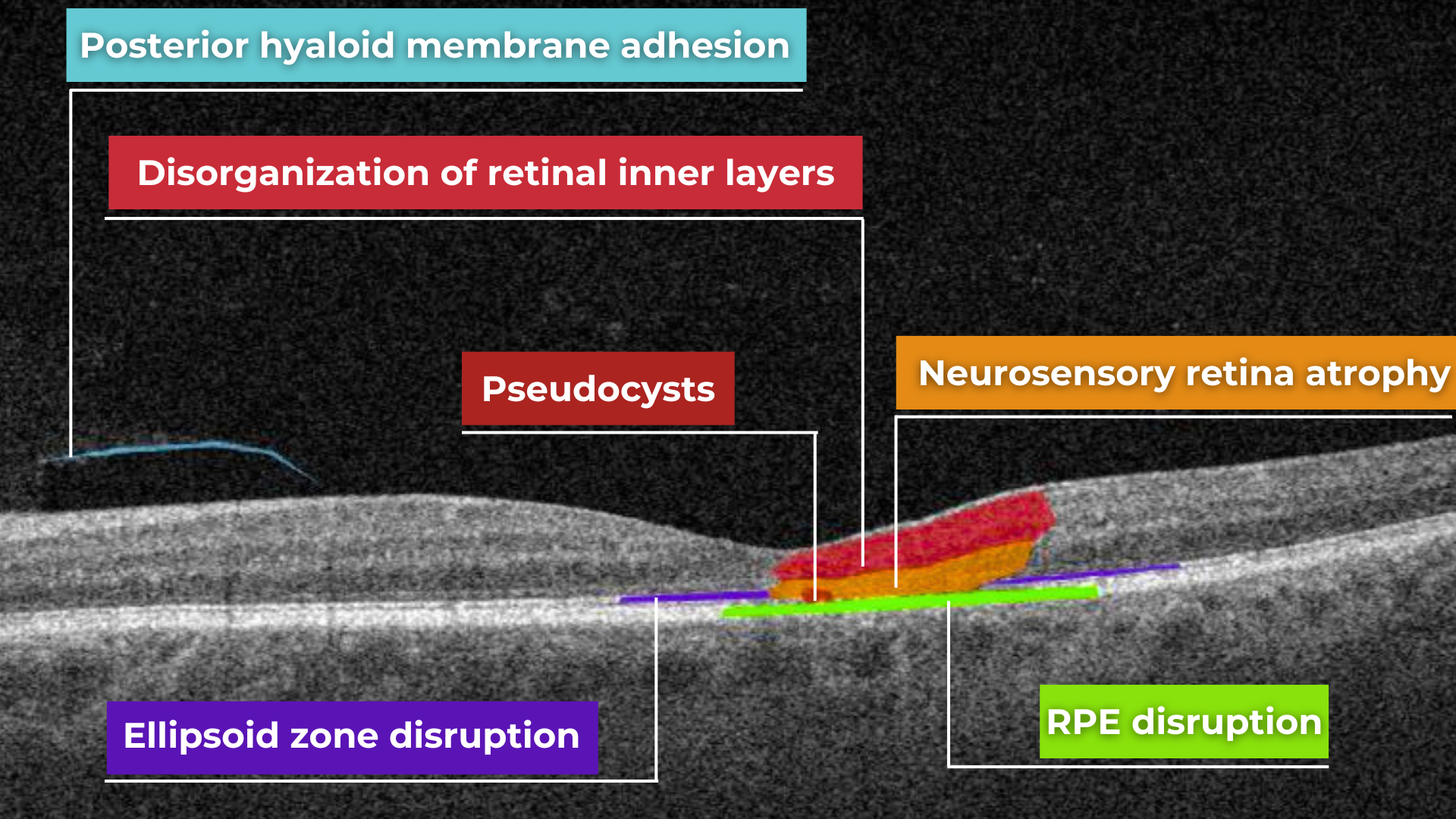
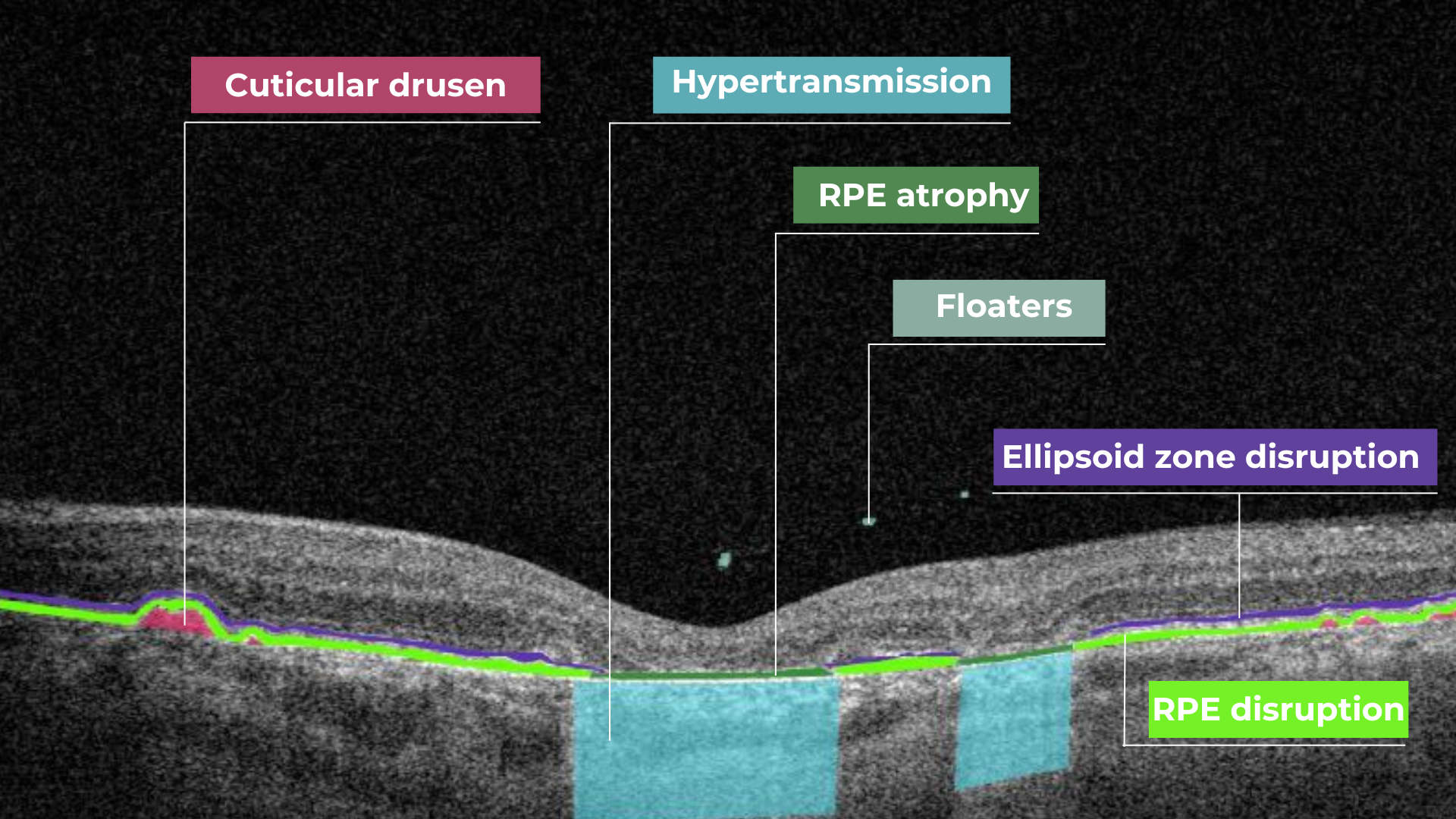 Hypertransmission on OCT
Hypertransmission on OCT