Recently Posted
-

Technologies in Optometry: Clare and Illingwort & Altris
 Altris Inc.
3 min.3 min.
Altris Inc.
3 min.3 min.The Client: Clare and Illingworth, renowned leaders in the field of optometry located in the UK.
The problem: The need to speed up the process of OCT interpretation and unburden the optometry team.
The Solution: Clare and Illingworth have embraced cutting-edge technology to enhance their Optical Coherence Tomography (OCT) analysis workflow. The introduction of Altris AI at this optometry center marks a significant milestone in their commitment to providing high-quality services to patients.
According to one of the owners of the optometry center, Richard, “We are adding a new OCT to one of our practices and will benefit from some extra support with AI to speed up the interpretation of results and assist the busy Optometry team.”
Altris AI, a leading provider of artificial intelligence solutions for healthcare, specializes in developing algorithms and software applications that augment medical imaging analysis. The integration of Altris AI into the British Optometry Center’s OCT workflow brings forth a host of advantages, revolutionizing the way eye conditions are diagnosed and managed.
Technologies in Optometry and Ophthalmology: How AI Helps
One of the key benefits of Altris AI is its ability to automate and expedite the analysis of OCT scans. Traditionally, optometrists spent considerable time manually reviewing and interpreting OCT images.
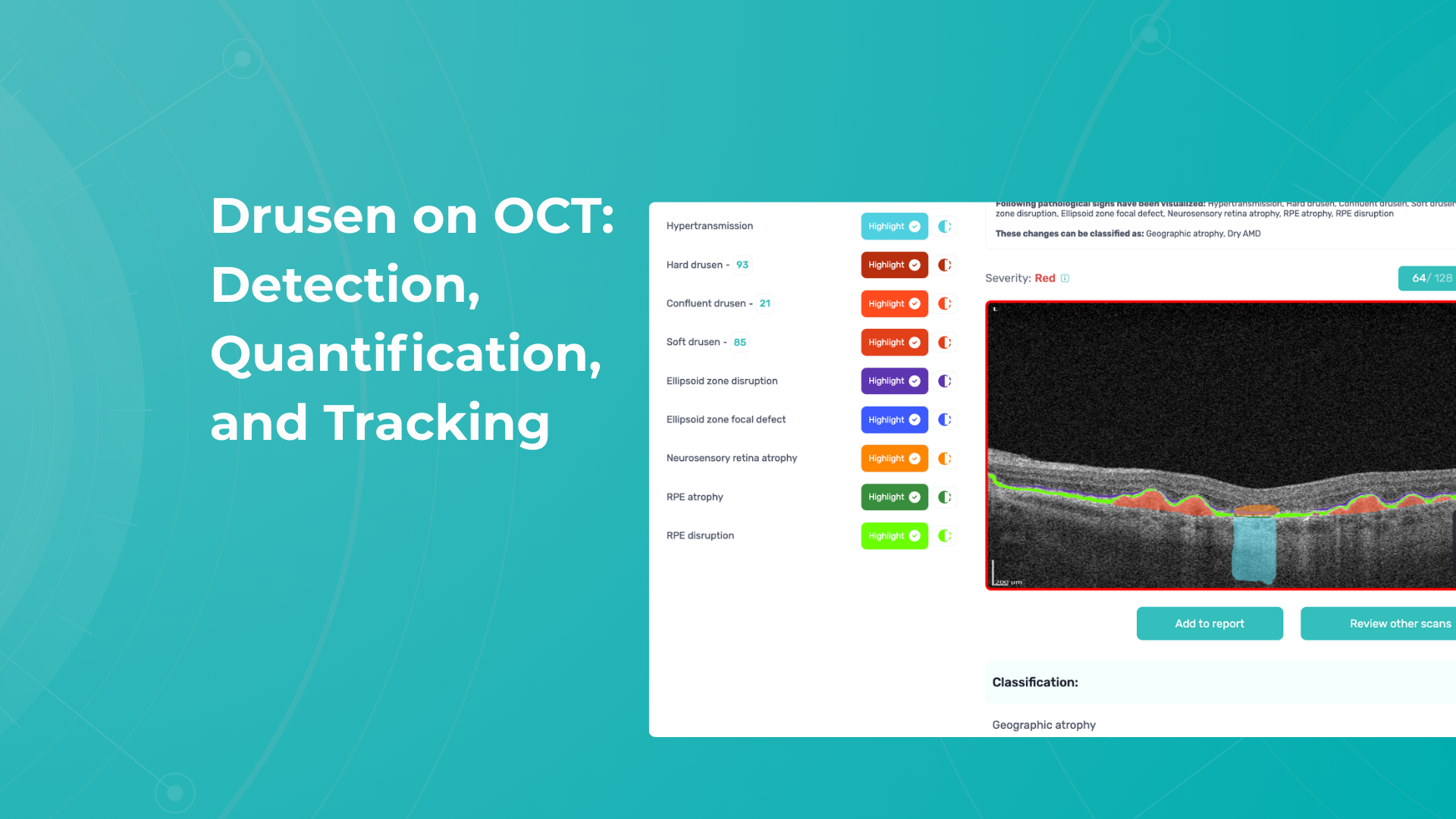
FDA-cleared Altris AI is created to make the OCT workflow more effective
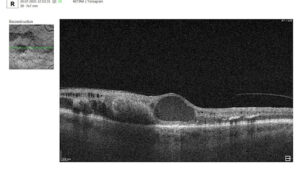
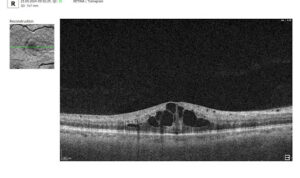
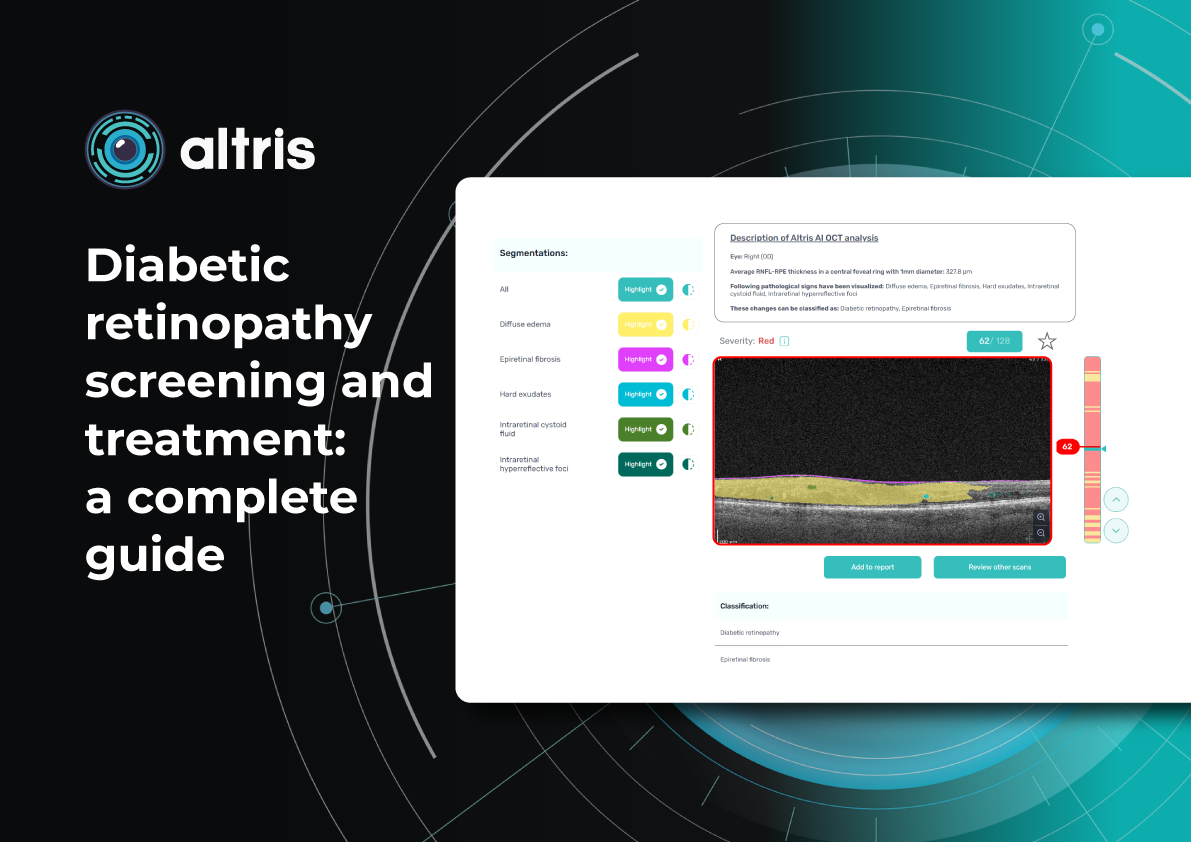
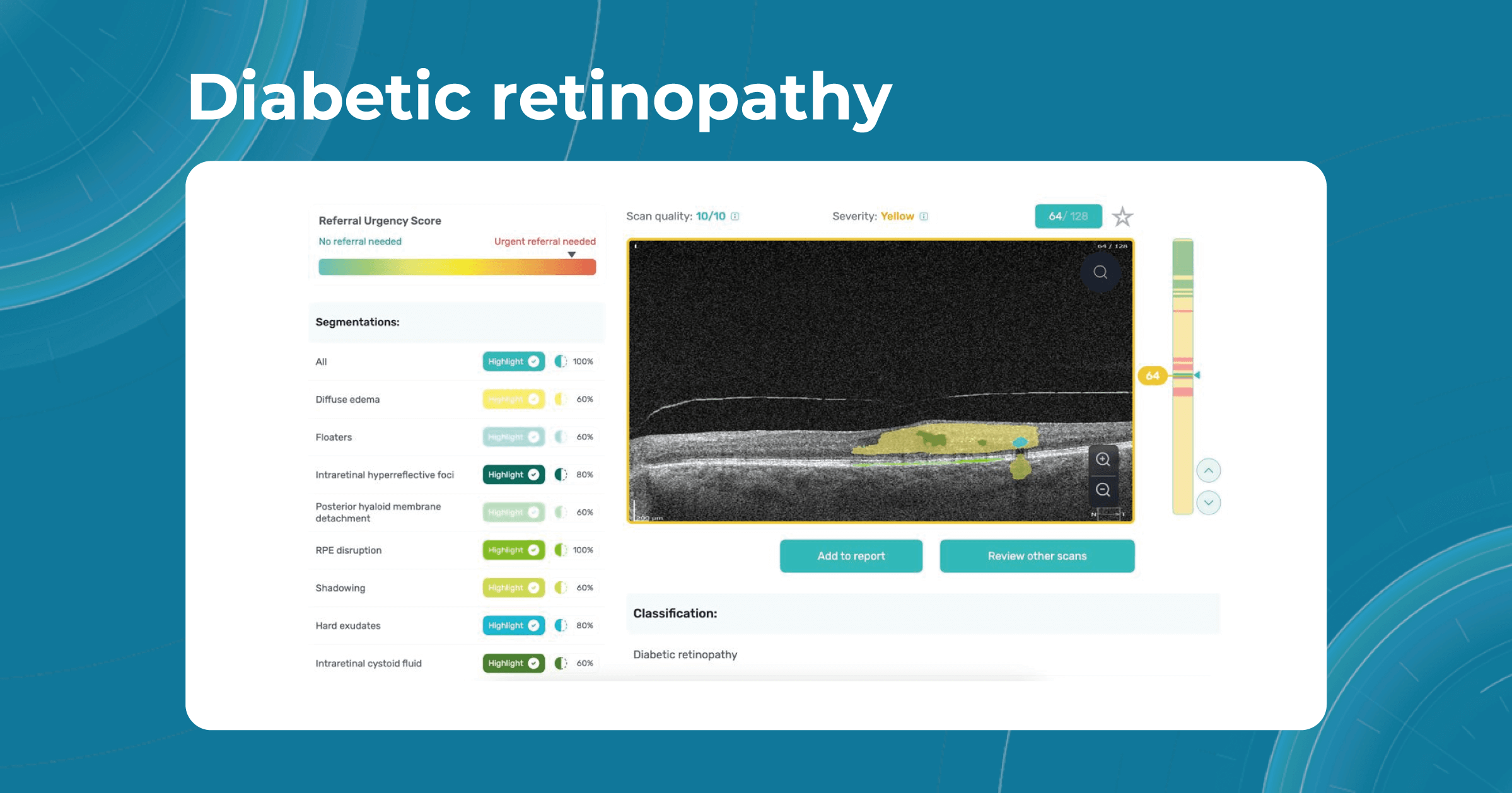
How does it work? Altris AI serves as a copilot, analyzing OCT scans in parallel to the eye care specialist. For instance, on this OCT scan, Altris AI detects Diffuse Edema, Floaters, Intraretinal Hyperreflective Foci, Posterior Hyaloid Membrane Detachment, RPE disruption, Shadowing, Hard Exudates, Intraretinal Cystoid Fluid.
- The classification in this case would be Diabetic Retinopathy.

With Altris AI, the process becomes significantly faster and more efficient. The AI algorithms can quickly analyze intricate details within the scans, providing clinicians with accurate and timely insights into the patient’s eye health.
Moreover, the use of Altris AI contributes to increased diagnostic accuracy. The algorithms are trained on vast datasets, learning to recognize subtle patterns and anomalies that may escape the human eye.
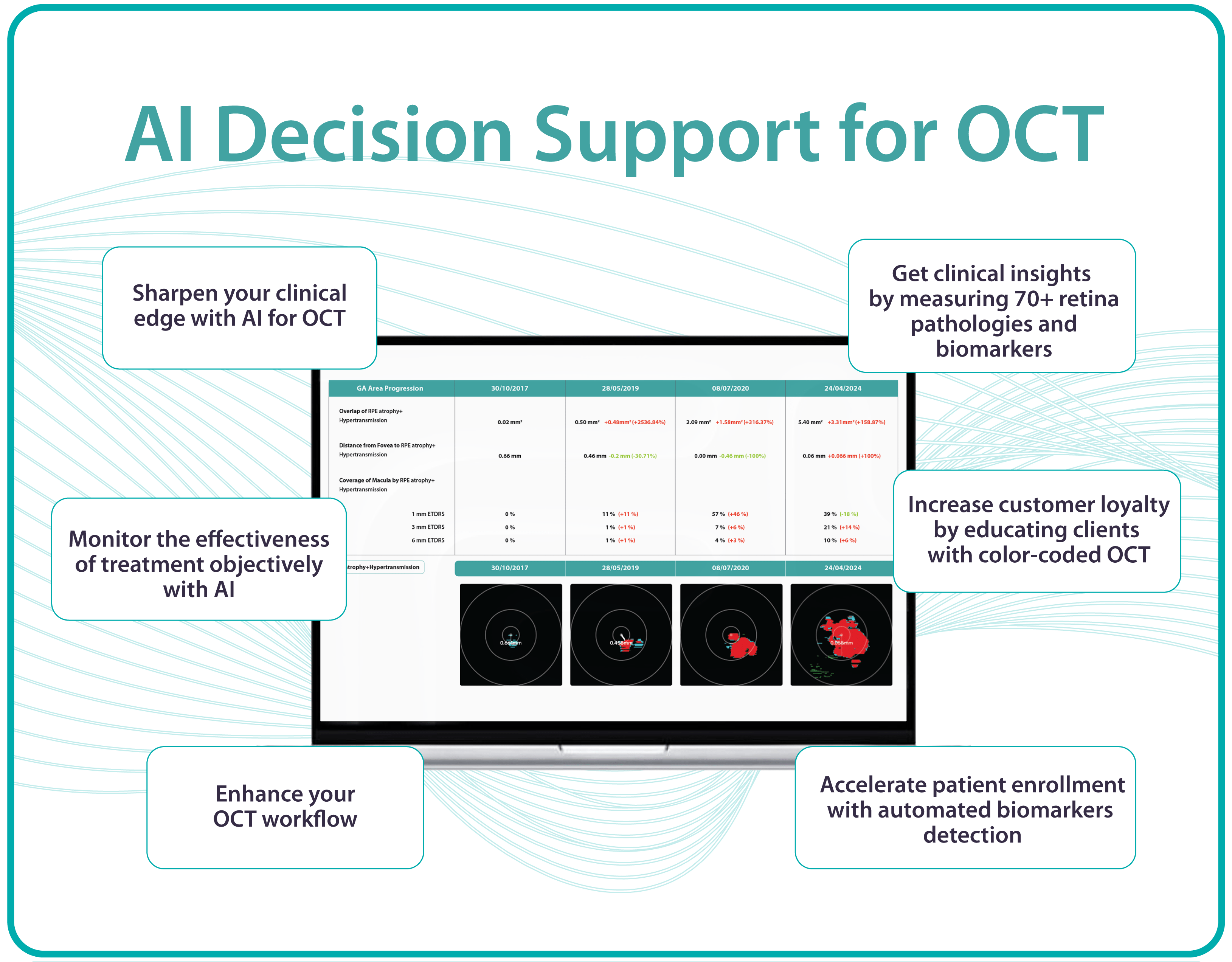
Thus, Altris AI recognizes 70+ retina pathologies and biomarkers, including DME, DR, GA, AMD, etc.
Technologies in Optometry are paving the way to a new future where eye care specialists and AI will work together for better patient outcomes. AI will never be able to substitute eye care specialists because the final diagnosis must include clinical history, results of lab tests, and other diagnostic methods.
Disclaimer: USA FDA 510(k) Class II; Altris Image Management System (Altris IMS); AI/ML models and components intended to use for research purposes only, not for clinical diagnosis purposes.
-

OCT Layers of Retina
 Maria Martynova
5 min.5 min.
Maria Martynova
5 min.5 min.OCT Layers of Retina: modern approach to segmentation
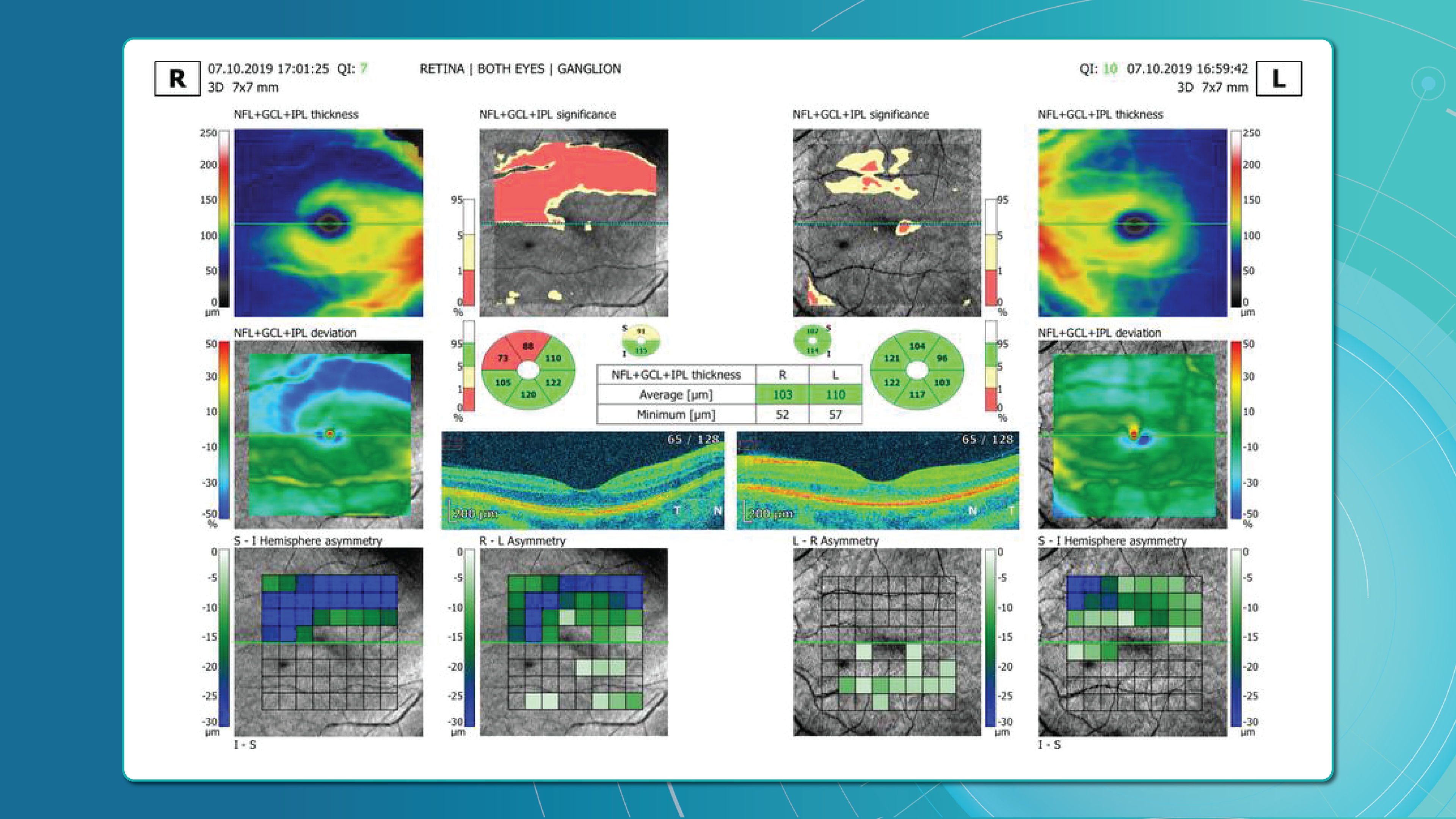
The knowledge about macular retinal layer thicknesses and volume is an important diagnostic tool for any eye care professional today. The information about the macular retinal layers often correlates with the evaluation of severity in many pathologies.
Manual segmentation is extremely time-consuming and prone to numerous errors, which is why OCT equipment manufacturers use automatic macular retinal layer thickness segmentation.
Yet, retina layer segmentation in different OCT equipment manufacturers as well as in different OCT models varies significantly. It is sometimes difficult even for an experienced ECP to find the correlations and track the pathology dynamics. The normative bases refer only to the thickness of the entire retina, they are not related to segmentation. However, if the segmentation is performed incorrectly by the machine, it will lead to an incorrect calculation of the thickness of the retina or its layers, and then the assessment will be incorrect.
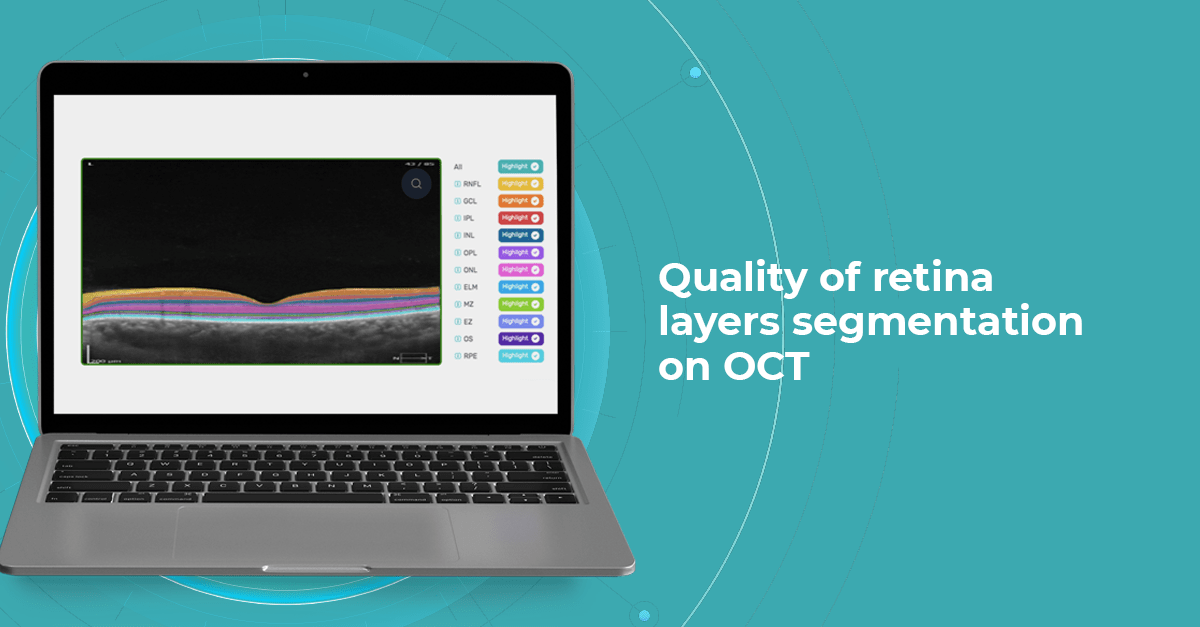
At Altris AI we aim to visualize retina layers for a more accurate understanding of pathological process localization. Such retina layers segmentation allows for defining the localization of the pathological process and tracing in dynamics the spread of the pathological process or the aftermath in the retina structure after its completion.
For instance, the EZ layer is important for vision loss forecasting.
OCT Manufacturers & Retina Layers Analysis
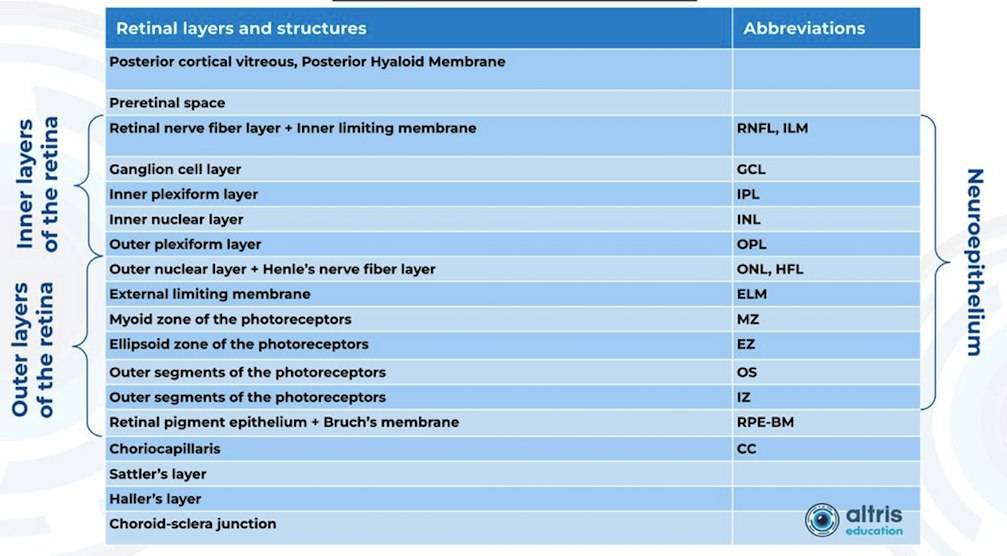
From 2010 most eye care specialists have used the same OCT International Nomenclature for Optical Coherence Tomography. OCT equipment manufacturers rely on this nomenclature for retina layer thickness calculation and most ophthalmologists use it as well. Here is a variant of OCT layer segmentation:

Taking into account retina structure, some layers can be united into complexes. For instance, the ganglion complex includes RNFL, ganglion cell layer & OPL.
Let’s take a look at various OCT equipment manufacturers and the way they perform retina layer segmentation analysis.
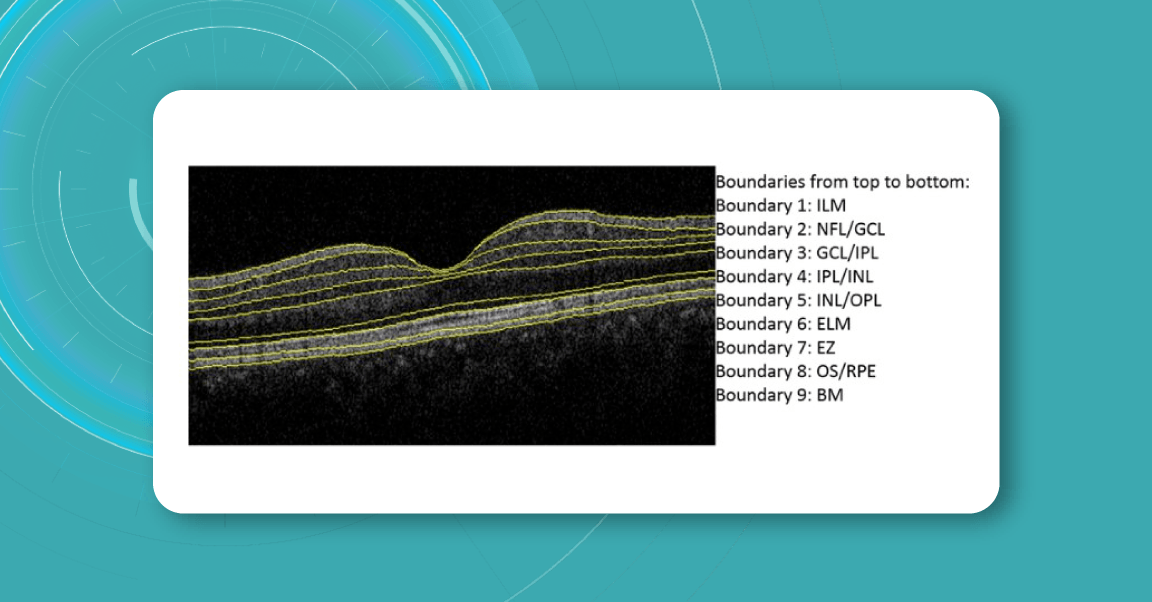
For instance, here is how Topcon Advanced Boundary Segmentation (TABSTM) automated segmentation differentiates between nine intraretinal boundaries:
- ILM
- NFL/GCL,
- GCL/IPL,
- IPL/INL,
- INL/OPL,
- ELM
- EZ
- OS/RPE
- BM

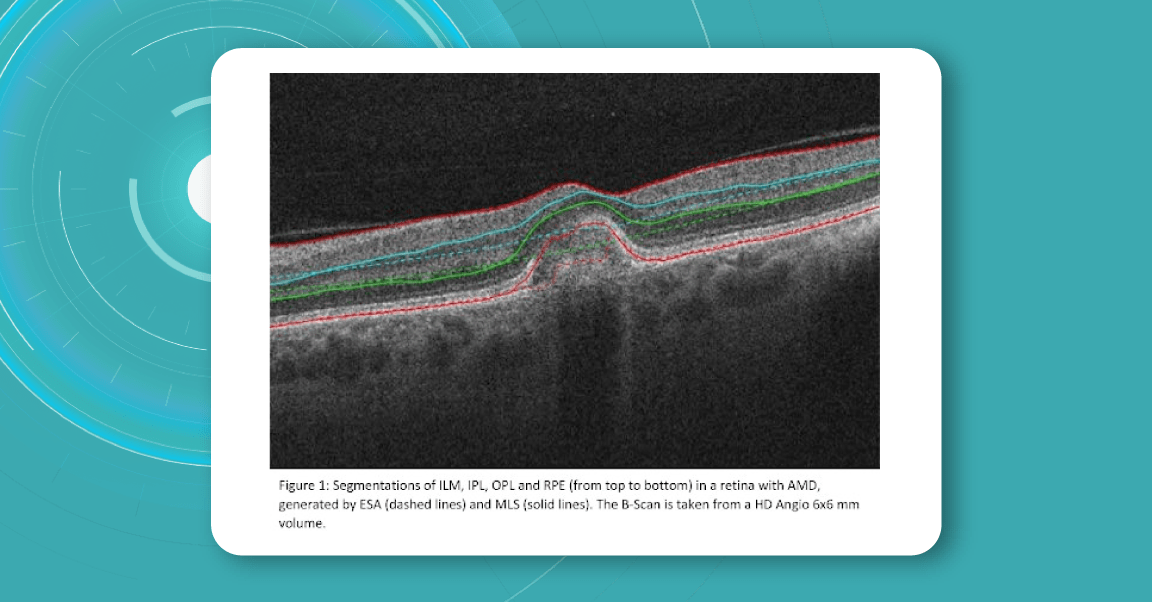
Zeiss CIRRUS uses two approaches to retina layer segmentation.

The existing segmentation algorithm (ESA) in CIRRUS estimates the positions of the inner plexiform layer (IPL) and outer plexiform layer (OPL) based on the internal limiting membrane (ILM) and retinal pigment epithelium (RPE). To improve the accuracy of the segmentation of these layers, a multi-layer segmentation algorithm (MLS) was introduced, it truly segments layers instead of estimating their position.
Heidelberg Engineering offers to learn about the following inner and outer retina layers on their website. There are 10 retina layers according to Heidelberg, and they are the following:
- ILM
- RNFL
- GCL
- IPL
- INL
- OPL
- ONL
- ELM
- PR
- RPE
- BM
- CC
- CS
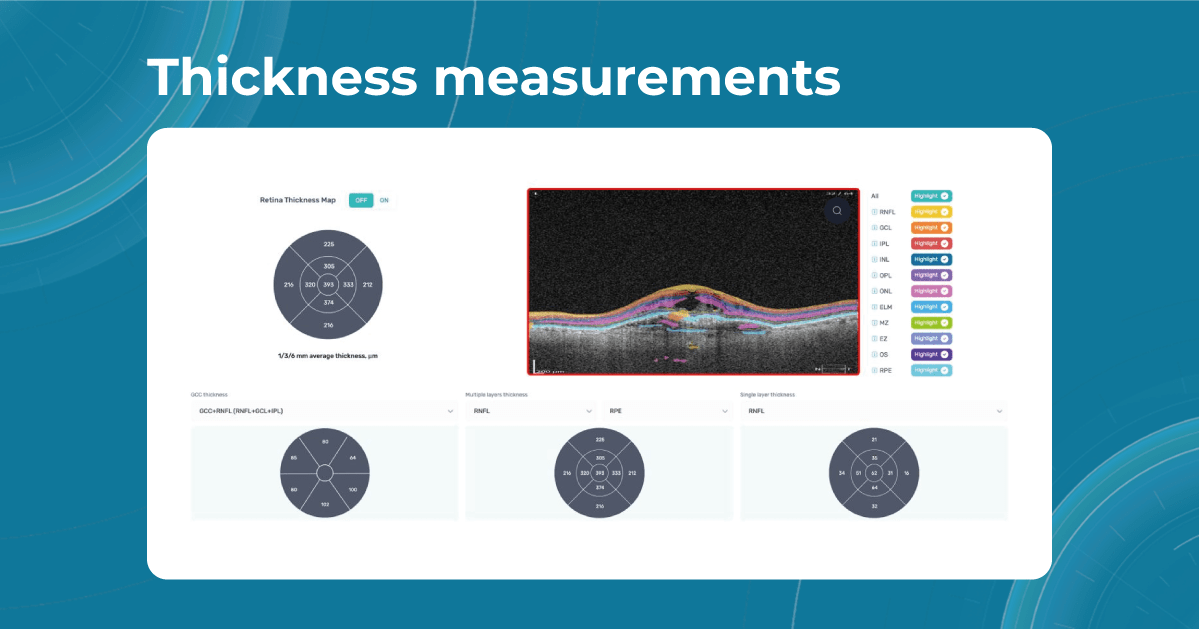
Retina Layers on OCT with Altris AI: More Clinical Insights
Altris AI segments 12 retina layers and measures their thickness with maximum precision. Here are the OCT retina layers we work with:
-
RNFL – Retinal Nerve Fiber Layer
Contains ganglion cell axons; thinning is a key marker for glaucoma.
Measuring its thickness helps detect and monitor glaucomatous damage. -
GCL – Ganglion Cell Layer
Composed of ganglion cell bodies; damage here indicates neurodegeneration.
Thickness assessment aids in the early diagnosis of glaucoma and optic neuropathies. -
IPL – Inner Plexiform Layer
The site of synapses between bipolar and ganglion cells is vital for signal relay.
Changes in thickness can reflect inner retinal dysfunction, especially in diabetic retinopathy. -
INL – Inner Nuclear Layer
Houses bipolar, amacrine, and horizontal cell bodies are essential for visual processing.
Swelling or thinning may indicate retinal vascular disease or macular edema. -
OPL – Outer Plexiform Layer
Where photoreceptors connect to bipolar cells; disruptions may signal early maculopathy.
Thickness alterations can be associated with retinal ischemia or structural disorganization. -
ONL – Outer Nuclear Layer
Contains the nuclei of photoreceptors; thinning may indicate photoreceptor loss.
Tracking its thickness supports evaluation of photoreceptor integrity in degenerative diseases. -
ELM – External Limiting Membrane
A structural boundary supporting photoreceptor alignment and health.
Integrity and thickness are indicators of photoreceptor viability in macular disorders. -
MZ – Myoid Zone of Photoreceptors
Contains organelles like the endoplasmic reticulum; changes may reflect early photoreceptor stress.
Subtle thickness variations may serve as early markers of photoreceptor damage. -
EZ – Ellipsoid Zone of Photoreceptors
A mitochondrial-rich layer critical for photoreceptor energy supply; disruption suggests dysfunction.
Its thickness and continuity are key indicators of visual potential and retinal health. -
OS – Outer Segment
Responsible for light detection; damage here impairs visual transduction.
Measuring OS thickness is essential for assessing photoreceptor function and recovery. -
RPE – Retinal Pigment Epithelium
Supports photoreceptors and waste removal; essential in maintaining retinal health.
Changes in RPE thickness can indicate AMD, central serous chorioretinopathy, and other retinal diseases. -
BM – Bruch’s Membrane
A barrier beneath the RPE; thickening or breaks are early signs of AMD.
Assessing thickness helps detect early signs of age-related macular degeneration and choroidal changes.
Why is accurate retina layer segmentation important?
Retina layers segmentation helps eye care professionals to understand which pathology to consider in the first turn. For instance, changes in RPE and PR signify the development of Macular Degeneration.
Often such changes can also inform eye care specialists about the development of pathologies that lead to blindness, such as glaucoma, AMD, and Diabetic Retinopathy.
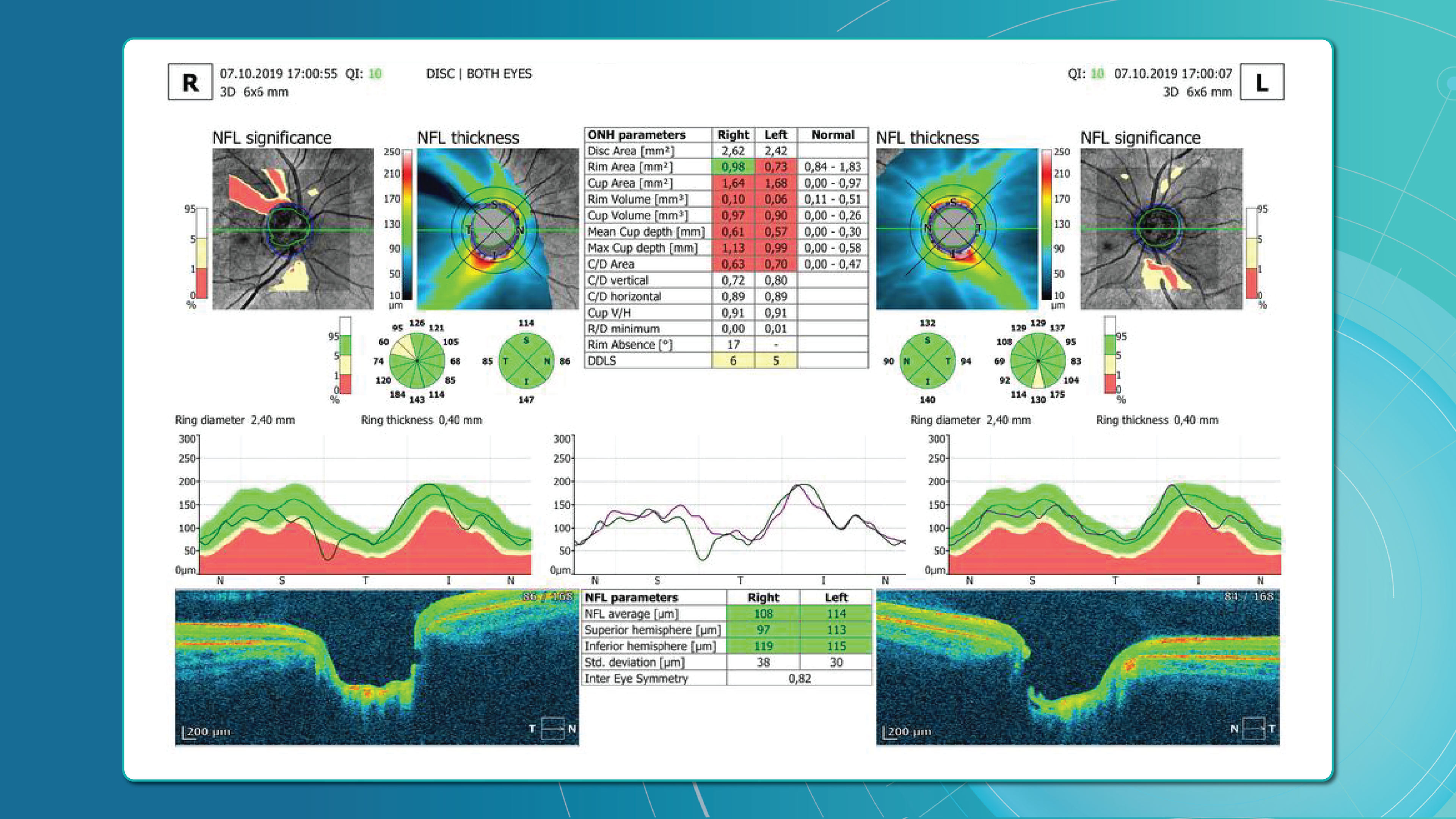
- Early Glaucoma Detection
Historically, evaluation of early glaucomatous change has focused mostly on optic disk changes. Modalities such as optical coherence tomography (OCT), confocal scanning laser ophthalmoscopy (HRT) or scanning laser polarimetry (GDx) with specially developed software algorithms have been used to quantitatively assess such changes. However, glaucomatous damage is primarily focused on retinal ganglion cells, which are particularly abundant in the peri-macular region (the only retinal area with a ganglion cell layer more than 1 layer thick), constituting, together with the nerve fiber layer, up to 35% of retinal macular thickness.
Therefore, glaucomatous changes causing ganglion cell death could potentially result in a reduction of retinal macular thickness. Indeed, by employing specially developed algorithms to analyze OCT scans, previous studies have reported that glaucoma, even during the early stage, results in the thinning of inner retinal layers at the macular region.
According to this study, the RNFL, GCL, and IPL levels out of all the retinal layers, the inner-most layers of the retina: the retinal nerve fiber layer (RNFL), ganglion cell layer (GCL), and inner plexiform layer (IPL) show the best discriminative power for glaucoma detection. Among these, the RNFL around the circumpapillary region has shown great potential for discrimination. The automatic detection and segmentation of these layers can be approached with different classical digital image processing techniques.
- Detection of AMD
This first population-based study on spectral-domain optical coherence tomography-derived retinal layer thicknesses in a total of ∼1,000 individuals provides insights into the reliability of auto-segmentation and layer-specific reference values for an older population.
The findings showed a difference in thicknesses between early AMD and no AMD for some retinal layers, suggesting these as potential imaging biomarkers. When comparing layer thicknesses between early AMD and no AMD (822 eyes, 449 participants), the retinal pigment epithelium/Bruch’s membrane complex demonstrated a statistically significant thickening, and photoreceptor layers showed a significant thinning.
- Detection of DR
The depth and spatially resolved retinal thickness and reflectance measurements are potential biomarkers for the assessment and monitoring of Diabetic Retinopathy, one of the key reasons for blindness around the globe.
For instance, this study confirmed that decreased RNFL thickness and increased INL/OPL thickness in diabetics without DR or with initial DR suggest early alterations in the inner retina. On the contrary, the outer retina seems not to be affected at the early stages of DM. Automatic intraretinal layering by SD-OCT may be a useful tool to diagnose and monitor early intraretinal changes in DR.
Conclusion:
OCT layer segmentation is crucial for the accurate detection of pathologies in the eye, especially in the field of ophthalmology and medical imaging. Here are several reasons why it is important:
Precise Diagnosis: Retina layer segmentation provides a detailed map of the different retinal layers, which helps in the precise diagnosis of various eye conditions. It allows clinicians to identify the exact location of abnormalities, such as cysts, hemorrhages, or lesions, within the retina.
Quantitative Analysis: It enables quantitative analysis of retinal structures. By measuring the thickness, volume, and other characteristics of specific layers, clinicians can assess the severity and progression of diseases like diabetic retinopathy, macular degeneration, and glaucoma.
Early Detection: Some retinal pathologies manifest in specific layers of the retina before becoming visible on a fundus photograph. Retina layer segmentation can help detect these changes at an early stage, potentially leading to earlier intervention and improved outcomes.
Treatment Planning: Knowing the precise location of pathologies within the retina’s layers can aid in the planning of treatment strategies. For example, in cases of macular holes or retinal detachment, surgeons can use this information to guide their procedures.
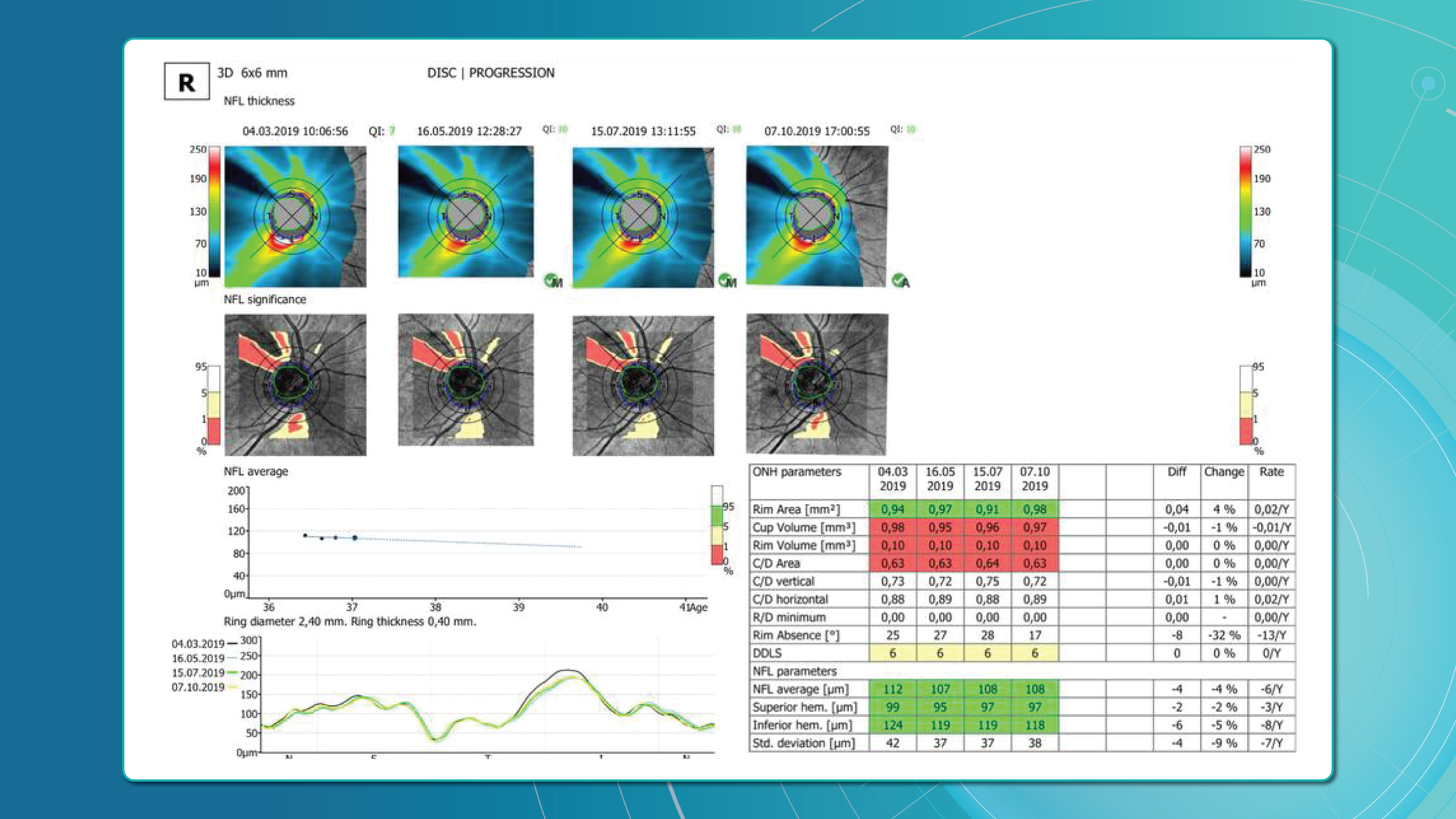
Monitoring Disease Progression: Retina layer segmentation is valuable for monitoring how retinal diseases progress over time. Changes in the thickness or integrity of specific layers can be tracked to assess the effectiveness of treatments or the worsening of conditions.
Disclaimer: USA FDA 510(k) Class II; Altris Image Management System (Altris IMS); AI/ML models and components intended to use for research purposes only, not for clinical diagnosis purposes.
-

Altris AI for Buckingham and Hickson Optometry, the UK
 Altris Inc.
1 min.
Altris Inc.
1 min.Business case: Altris AI for Buckingham and Hickson Optometrists
The Client: Buckingham and Hickson is a family-run optometry practice that was established in 1960 in the United Kingdom. The optometry practice offers a number of services:
- Wide range of spectacle frames and lenses.
- Contact lenses.
- Glaucoma referral refinement.
- Cataract choice referral.
- OCT examination.
- NHS and private eye tests.
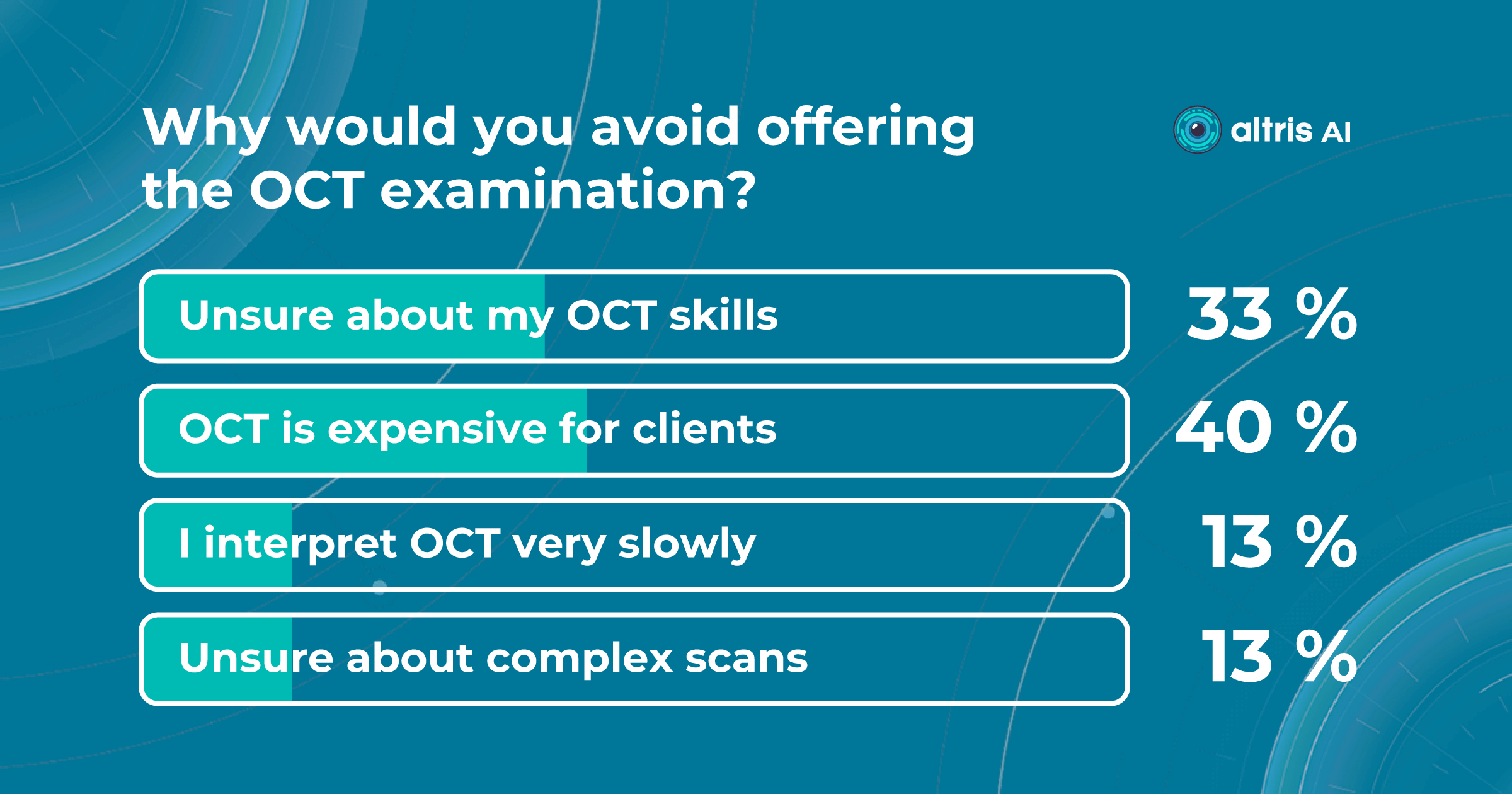
The challenge: The optometry owners wanted to test how Artificial Intelligence can assist them in OCT examination or, to be more precise, in providing a second opinion for OCT scans.
OCT examination is one of the best retina diagnostics methods, however in many cases OCT scan interpretation can be really challenging for several reasons:
- Variability in Anatomy: There is significant natural anatomical variation among individuals. What may be considered normal for one person may be abnormal for another. Eye care specialists need to account for these variations when interpreting OCT scans, but this often requires years of experience.
- Various Eye Conditions: Eye care specialists use OCT scans to diagnose and monitor a wide range of eye conditions, including macular degeneration, diabetic retinopathy, and retinal detachment, among others. Each of these conditions can manifest in different ways on OCT scans, making interpretation challenging.
- Progression Monitoring: Ophthalmologists often use OCT to monitor disease progression and the effectiveness of treatment. Tracking subtle changes over time can be difficult, as it requires precise comparisons of multiple scans.
- Artifacts: OCT scans are susceptible to artifacts, such as shadowing, motion artifacts, and signal dropout, which can obscure or distort the image. Recognizing and mitigating these artifacts is essential for accurate interpretation.
- Experience and Training: Accurate interpretation of OCT scans in optometry and ophthalmology requires specialized training and experience.
- Evolving Technology: OCT technology continues to advance, introducing new techniques and capabilities. Staying current with these advancements and understanding their clinical implications is an ongoing challenge for ophthalmologists.
-

AI for OCT analysis in optometry chains: 8 Reasons to invest
 Mark Braddon
5 min.
Mark Braddon
5 min.AI for OCT analysis in optometry chains
Optometry chains offer a wide range of eye care services, making it convenient for patients to access eye care locally.
However, the widespread accessibility of optometry chains has a reverse side for them. The shortage of employees, new unfamiliar equipment for diagnostics, and a large number of patients create an extremely challenging workflow for many optometrists. This, in turn, creates a number of challenges that can be more familiar to Optometry chains: low optometrist recruitment and retention, inconsistent quality of examination throughout the practices, lack of communication with patients, etc.
Automation of routine processes and digitalization have always served as answers to challenges like these in any industry, and healthcare is no exception. Luckily, automation of one of the most complex tasks for optometrists – OCT examination is already available to optometry chains with Artificial Intelligence (AI).
OCT proves to be one of the most efficient diagnostic tools for many modern top-notch optometry practices, however, mastering it requires skills and time. Artificial intelligence tools, such as AI for OCT analysis platform, can automate many routine processes which will have enormous benefits for any optometry chain. The top 8 benefits are the following:
-
#1 AI for OCT increases clinical efficiencies
Automating OCT scan analysis through AI reduces the time optometrists spend on image interpretation. This allows optometrists to focus on more complex cases, patient interactions, and personalized treatment plans. For any large optometry chain, saving time means providing more patients with high-quality service.
How does it work in practice?
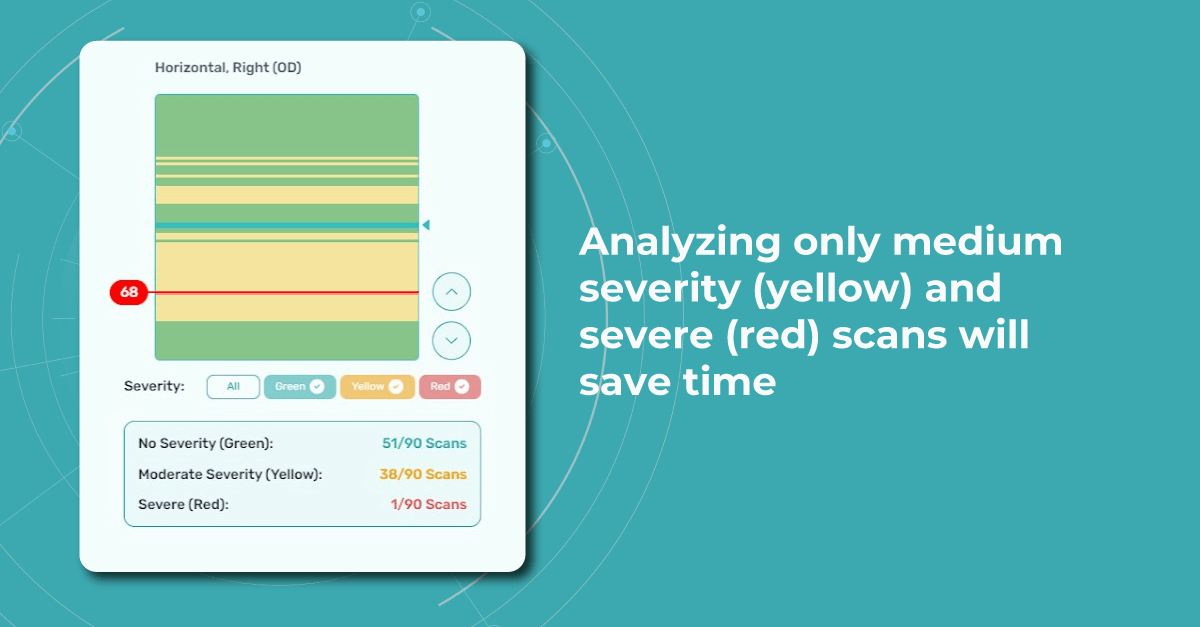
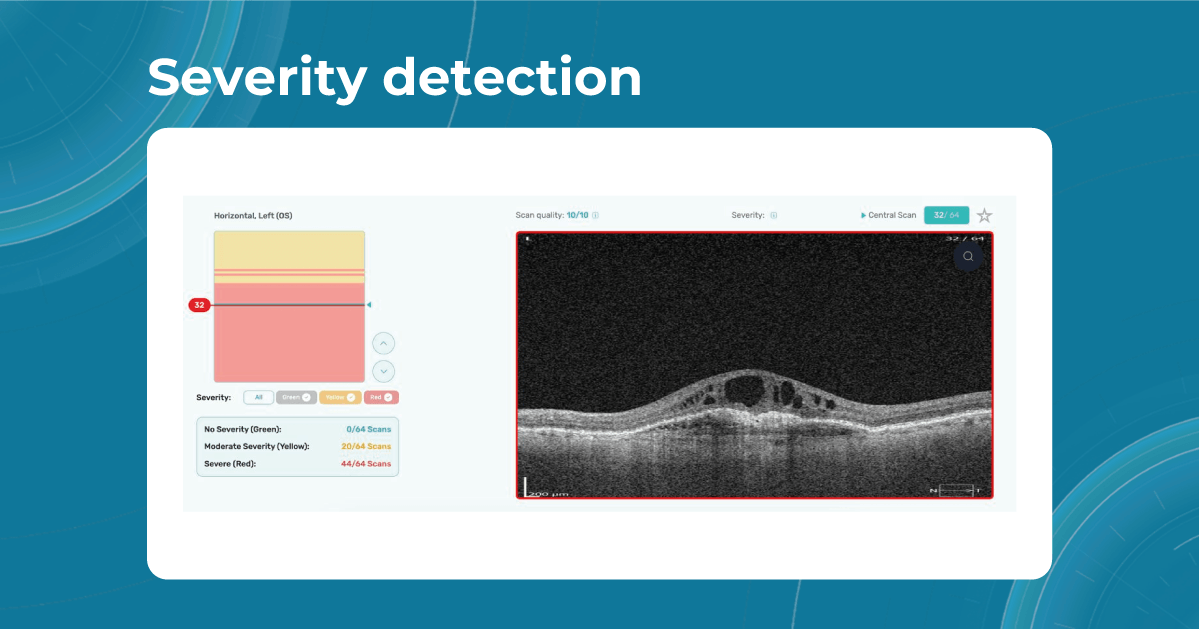
For instance, Altris AI has a severity grading of b-scans. Severity grading means that it is easy to see if the eye is healthy (removing any need to spend time interpreting) or highlight where the pathology is and the degree of severity.
- Green- no pathology detected
- Yellow- mild to medium level of severity
- Red – severe pathology detected

-
#2 AI for OCT provides consistently high standard of quality throughout the chain
AI algorithms provide consistent and standardized analysis regardless of the individual interpreting OCT scans. This reduces variability in diagnoses and ensures that patients receive uniform care across different clinics and practitioners within the optometry chain.
AI algorithms can analyze OCT scans with incredible precision and consistency. They can detect subtle changes in retinal structures that might be missed by human observers, leading to earlier and more accurate diagnoses of various eye conditions such as macular degeneration, glaucoma, diabetic retinopathy, and more.
This will help younger less experienced optometrists and will serve as a second opinion tool for more experienced specialists.
-
#3 AI for OCT enables better retention of employees
The shortage of optometrists in the world is staggering. 14 million optometry specialists are needed worldwide according to the WHO, while today there are only 331K ready to work.
It is equally difficult to hire and retain a good optometrist for a company in 2023. However, more and more young optometrists choose innovative businesses that use technology to improve the workflow. Top-notch equipment, convenient scheduling tools, and of course, Artificial Intelligence for OCT & fundus photo analysis might be the perks that will help optometrists to choose your optometry business.

Fresh from college optometrists feel more confident when they know that they will have a backup when reviewing OCT scans
-
#4 Reduced Workload Burden
Optometrists often have heavy workloads, and AI can help alleviate some of this burden by handling routine tasks like initial image analysis. This enables optometrists to spend more time on patient consultations and treatment planning.
According to a survey by the General Optical Council, 57% of optometrists worked beyond their hours in 2022. Optometrists were more likely to be working beyond their hours (60%) or finding it difficult to provide patients with the sufficient level of care they needed (34%) when compared to other registration types.
It is possible to outsource preliminary image analysis to Artificial Intelligence tools but communication and empathy are human tasks only.
-
# 5 AI promotes enhanced patient education
Let’s not forget about the patients. AI-generated OCT reports can help explain complex medical conditions to patients in a more understandable, visual way. After all 80% of all the information we receive is visual: imagine your optometrists not only telling but also showing what is going on with patients.
Comprehensive, color-coded OCT reports may improve patient education and engagement, leading to better treatment adherence and loyalty.
When patients don’t understand what they are paying for they are not likely to return for annual checkups. At Altris AI we created smart OCT reports that are comprehensible for patients as well as optometrists. We visualize all the pathologies and the patients can trace the dynamics of
#6 Reducing a clinical risk. No chances of getting a legal inquiry because of a pathology missed
Optometry chains can perform around 40K OCT scans a week. Statistically speaking, the chance of missing a minor early pathology is huge simply because of the big number.
With the double-check that AI for OCT scan analysis provides, It is not possible to wipe the risk out for 100%, but it is possible to diminish the risk to the absolute minimum.
For the optometry chain, it might mean no bad PR and weird stories in the papers and subsequently, a better brand image.
-
#7 AI makes early detection of pathologies possible on OCT
AI algorithms can identify early signs of eye diseases that might not be easily recognizable in their early stage. This early detection can lead to timely interventions, preventing or minimizing patient vision loss.
Glaucoma, Wet AMD, Diabetic Retinopathy, and genetic diseases are among the pathologies that lead to blindness if not detected in time. Detecting pathological signs and pathologies related to these disorders in time can literally save patients from future blindness.
Early detection of pathologies means that it is possible to stop or reduce the risk of total blindness which is the best result in any sense. Early detection will allow optometrists to give valid recommendations, and advise on dieting and supplements right at the optical store.
-
#8 Competitive Edge
AI is a buzzword, and it’s not accidental. All major players understand its enormous value and invest in it. During the last presentation, the CEO of Google said “AI” 140 times, and let’s be honest, it is not to show off. It is because AI can actually make changes in business: automation of repetitive processes, workflow optimization, and human error reduction.
Adopting AI technology for OCT analysis showcases the optometry chain’s commitment to staying at the forefront of technological advancements in healthcare. Gaining a real competitive edge is another big goal.
This can attract patients who value cutting-edge approaches to diagnosis and treatment. A younger generation of patients are curious about new technologies, and this can be an additional lead magnet for them.

Conclusion
Incorporating AI for OCT analysis into optometry chains can enhance patient outcomes, make the workflow more efficient, and improve the performance of each optometry center. However, it’s important to ensure that the AI systems are properly validated, integrated into clinical workflows, and monitored to maintain their accuracy and effectiveness. More than that, it should complement, not replace, the expertise of optometrists. The technology should be used as a tool to aid optometrists and make OCT examination more effective.
Disclaimer: USA FDA 510(k) Class II; Altris Image Management System (Altris IMS); AI/ML models and components intended to use for research purposes only, not for clinical diagnosis purposes.
-
-

Normative Database in OCT: Limitations and AI Solutions
 Maria Martynova
06.09.20236 min read
Maria Martynova
06.09.20236 min readNormative Database in OCT: Limitations and AI Solutions
The first normative database for OCT was created in the early 2000s and were based on small studies of mostly white patients. However, as OCT technology has evolved, so too have the normative databases. Recent databases are larger and more diverse, reflecting the increasing ethnic and racial diversity of the population.
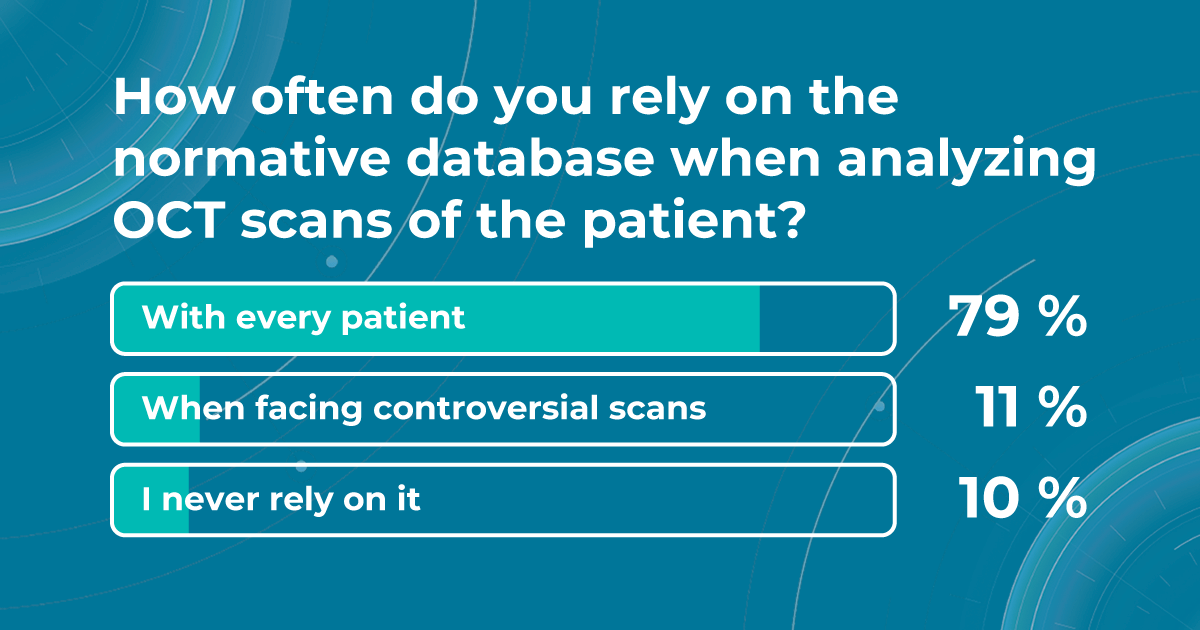
Nowadays, eye care specialists use normative database to compare the characteristics of a patient to a population-wide norm. This allows them to quickly and easily assess whether a patient’s retinal dimensions fall within normal limits. According to our survey, 79% of eye care specialists rely on the normative databases for OCT verdict with every patient.

However, despite the fact that normative databases are very widespread among specialists worldwide, they are not perfect. They can be affected by factors such as age, gender, axial length, and refractive error.
They can be influenced by low image quality due to different eye pathologies. It is essential to be aware of these limitations when interpreting normative data OCT parameters. That is why, in this article, we will discuss the benefits of the collaboration between AI decision-making tools and normative databases to improve patient outcomes.
What is a normative database for OCT
Before diving into the subject of the benefits and limitations of normative databases, we would like to remind you what a normative database is. From the moment of its invention, the OCT exam has rapidly gained widespread adoption and has become indispensable in the eye care practice. Critical to this success has been the ability of software to automatically produce important measurements, such as the thickness of the peripapillary retinal nerve fiber layer (RNFL) in tracking glaucoma progression or the total retinal thickness in the assessment of macular diseases.
In order to accurately interpret OCT scans, normative databases were created. These databases are now built into almost all commercial OCT devices, allowing eye care specialists to view colored reports and progression maps that assist in the rapid recognition and tracking of pathology.
Summing up, a normative database for OCT is a set of data that provides references for OCT thickness measurements in a healthy population. These databases are used to compare the OCT measurements of your patient to a population-wide norm.
Here are some of the OCT parameters that are commonly measured and compared to normative databases:
- Retinal nerve fiber layer (RNFL) thickness: the RNFL is a retinal layer that is measured around the optic nerve. This measurement is important for diagnosing optic nerve atrophy.
- Macular thickness: the macula is responsible for sharp central vision.
- Ganglion cell complex thickness: the ganglion cell complex is a group of cells in the retina that are responsible for transmitting visual information to the brain.
- Cup-to-disc ratio, neuroretinal rim, and other optic nerve parameters: are very important for diagnosing glaucoma and other optic nerve pathologies
These are just a few of the OCT parameters that are commonly measured in normative databases. The specific parameters that are measured can vary depending on the type of OCT device and the clinical application.
In addition, different OCT devices can have different measurement capabilities and resolutions. For example, a device that uses time-domain OCT (TD-OCT) technology may have a lower resolution than a device that uses spectral-domain or swept-source OCT (SD or SS-OCT) technology. This means that the normative database for a TD-OCT device may not be as accurate as the normative database for an SD or SS-OCT device.
What is more, the normative database for a particular device may be based on a specific population of patients. What are the benefits and limitations of normative databases?
Now that we have highlighted different aspects of the normative database definition let us discuss the benefits and limitations of this tool. Normative databases can sometimes be very helpful for eye care specialists in diagnosis, decision-making, and creating a treatment strategy for eye diseases such as glaucoma and macular degeneration.
- The measurement provided by the normative database can be used as a baseline for tracking a patient’s response to medication or other treatment. Eye care specialists can track changes between a few visits and determine the impact on the patient.
- Normative databases show deviations from the norm, which may be a reason for a more comprehensive examination.
- Eye care specialists can also use normative databases to compare the results of different OCT devices. This can help to ensure that they are using the most accurate device for their patients.
There are still challenges that must be overcome to develop normative databases sufficient for use in clinical trials. That is why current normative databases also have a lot of limitations.
Does not detect pathology
The normative database works only with the thickness of the retina and does not detect what is inside the retina. Therefore, it cannot detect all pathologies where there is no change in retinal thickness. In the early stages, these are absolutely all diseases. We can see deviations from the normative base only when the disease progresses to a later and more severe stage when the retinal thickness decreases or increases.
Limited diversity
Normative databases can be limited by factors like age, gender, and ethnicity of the population used to create them. This can result in reduced accuracy for patients who are not well-represented in the database.
Population variation
Even healthy patients can have some anatomical variations that fall within the range of normal. These variations may be falsely flagged as abnormalities when compared to the database.
How Altris AI platform can complement the information provided by the normative database
Normative databases in OCT play a crucial role in aiding diagnosis and treatment planning, but they also have limitations related to representation, disease progression, and data quality. Eye care specialists need to interpret the results in the context of the patient’s individual characteristics and other clinical information, using additional tools for scan interpretations.
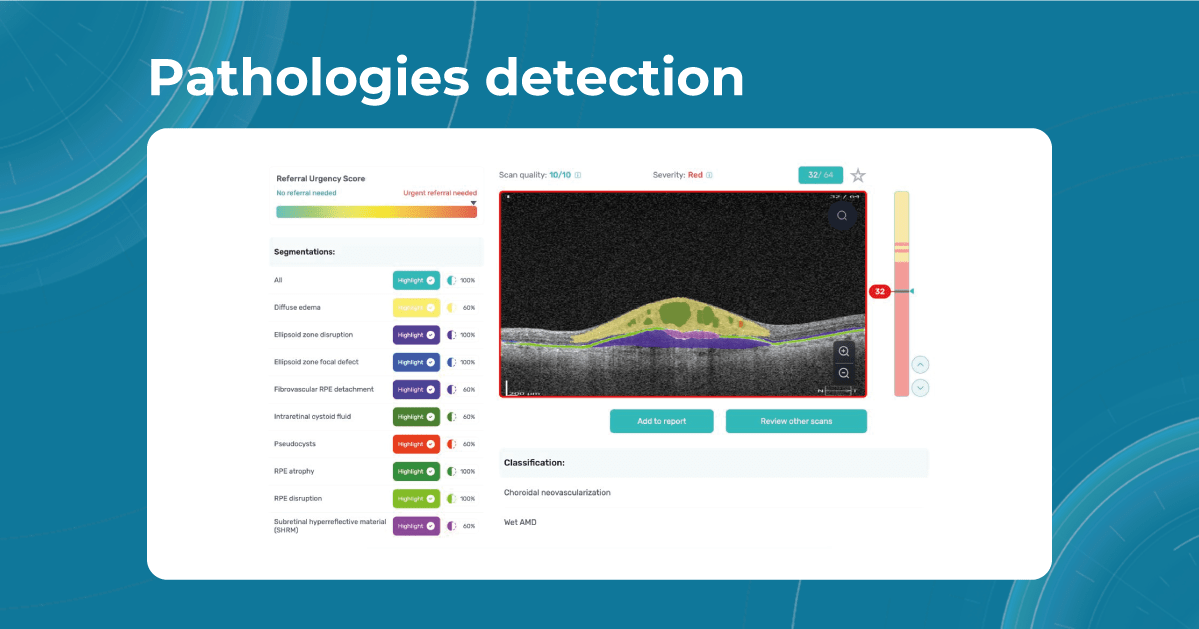
Sometimes, low-quality OCT scans can be inaccurately interpreted by the eye care specialist, and the normative database can showcase inaccurate measurements. Altris AI platform detects low-quality scans automatically and warns about the possibility of inaccurate results. In addition, the platform automates the detection of 70+ pathologies and pathological signs. Once the user uploads the scan, they can see visualized and highlighted pathological areas and pathology classification that the algorithm has detected. The user can also calculate the area and volume of detected biomarkers.

Artificial intelligence-based tools for OCT interpretation used along with normative databases can play a crucial role in clinical eye care. Altris AI, for example, can provide eye care specialists with additional and more precise information about separate retinal layer thickness. The system analyzes the thickness of each retina layer or several layers combined.

While normative databases provide information only about the thickness, AI tools equipped with deep learning models can detect pathological signs in OCT scans that might be missed by the normative database or the human eye, enhancing diagnostic accuracy. Altris AI algorithm classifies the OCT scans based on the degree of pathology found. It can distinguish green concern, which indicates normal retina, yellow – moderate with slight deviations, and red concern, which means high severity level.

Summing up
Despite their limitations, normative databases are an essential tool for the clinical use of OCT. They provide a valuable reference point for assessing patients and can help to identify some diseases. However, the normative database measures only the thickness, which is not enough to accurately diagnose the patient and create a treatment plan.
That is why incorporating AI into OCT interpretation streamlines the decision-making process. By automating the initial analysis of OCT scans, specialists can focus their attention on more complex cases, making the best use of their skills and experience. Moreover, embracing AI technologies empowers eye care specialists to personalize patient care with greater precision.
Disclaimer: USA FDA 510(k) Class II; Altris Image Management System (Altris IMS); AI/ML models and components intended to use for research purposes only, not for clinical diagnosis purposes.
-

AI Blindness Prevention: how AI can combat vision loss?
 Maria Martynova
07.08.20239 min read
Maria Martynova
07.08.20239 min readAI for Eye Diseases: how AI can combat vision loss?
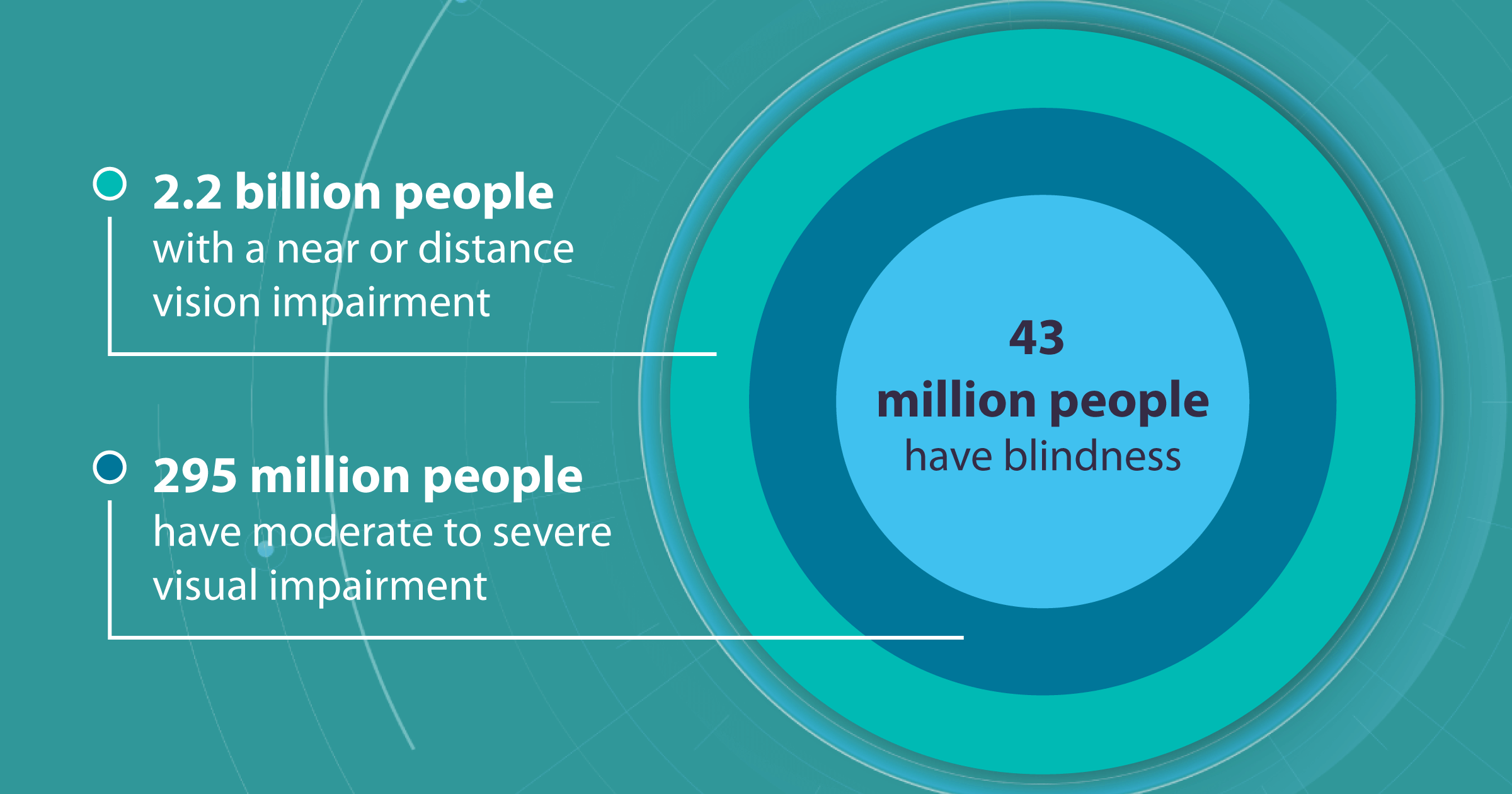
The total number of people with near or distant vision impairment reaches 2.2 billion worldwide.
Of these, 43 million people are blind, and 295 million are suffering from moderate to severe visual impairment. Although the numbers are constantly changing as new research is conducted, the global burden of blindness and visual impairment remains a significant problem of humanity in the fight against which specialists combine their forces with AI technologies.

AI blindness prevention tools are being actively developed to transform the landscape of vision care in many ways. Eye care specialists use AI systems for screening and detecting diseases that lead to vision loss. AI-powered smart monitors assist specialists in finding proper contact lenses and glasses. In addition, many researches are held with the help of AI algorithms, as they are able to process vast amounts of data.
In this article, we will discuss different applications of AI in blindness prevention, specifically how artificial intelligence tools can empower eye care specialists and extend beyond the clinical setting.
How to Prevent Blindness: Conditions and Risk Factors
Before talking about the developments in the AI sector toward blindness prevention, we would like to discuss the most common causes and risk factors of this impairment. Many health and lifestyle factors can influence the risk of vision loss. Smoking, excessive alcohol consumption, sun exposure, and poor nutrition can contribute to diseases that lead to vision loss.
In addition, there are many conditions that can lead to blindness if left with no proper treatment, among which are the following.
Age-related eye diseases
The global population is aging rapidly. The number of people aged 65 and over is projected to triple from 1 billion in 2020 to 2.1 billion in 2050. Considering this fact, age-related eye diseases have become a prominent cause of blindness. Such diseases as age-related macular degeneration (AMD), cataract, and glaucoma are more prevalent in older patients, and if left untreated, they can lead to fast and significant vision loss. Regular eye check-ups and timely interventions are crucial in managing these diseases and preventing severe visual impairment.

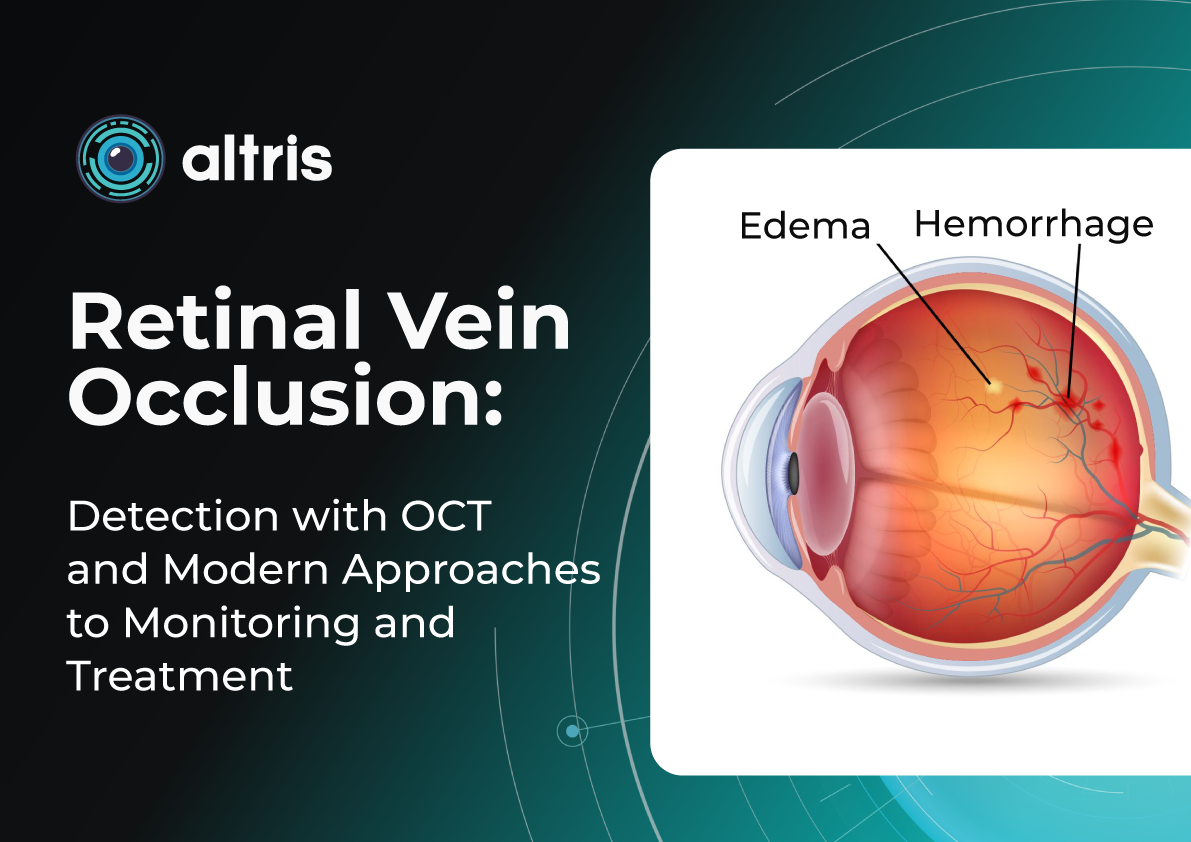
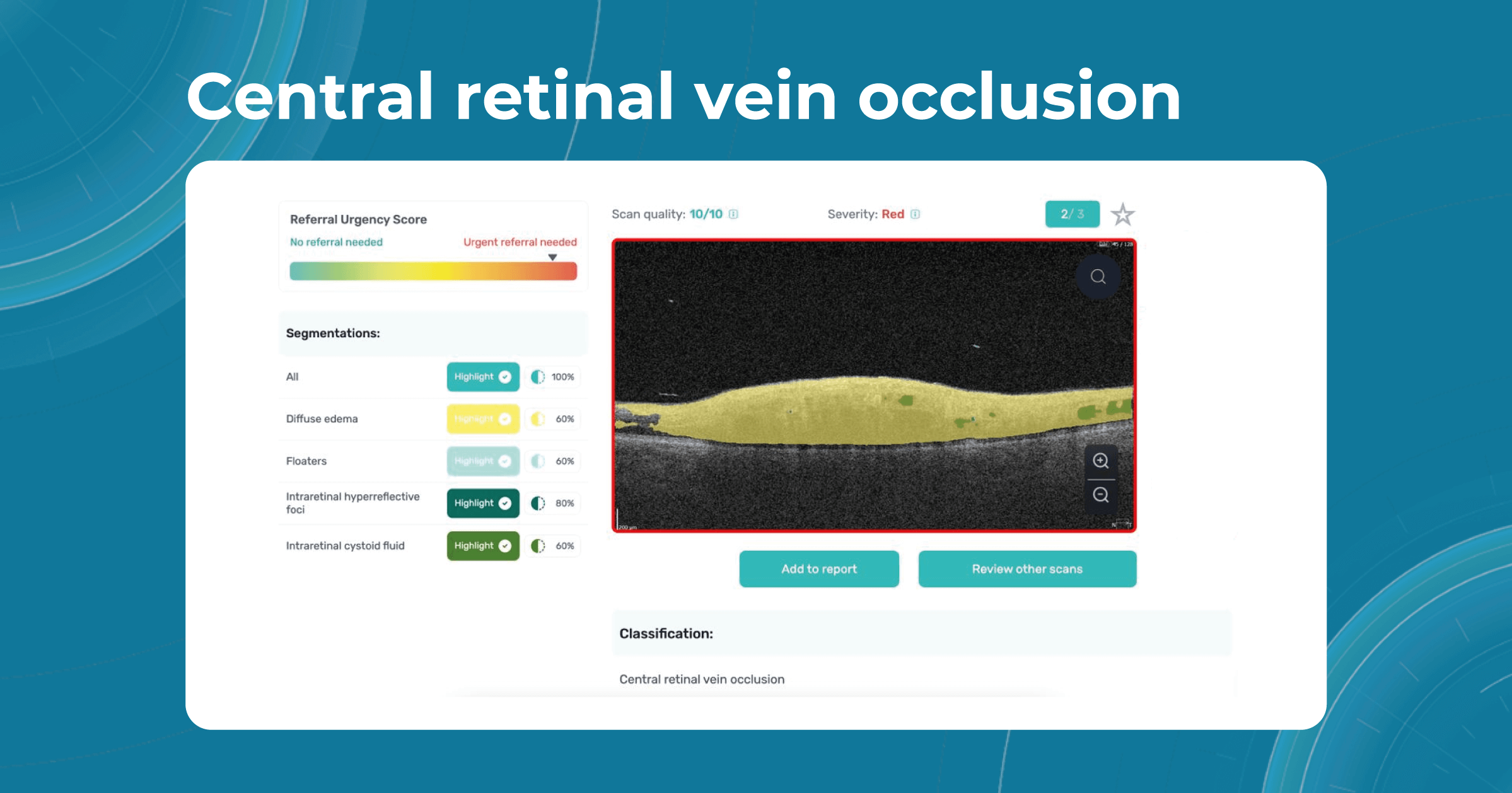
Besides AMD, there are a lot of age-related conditions which can be a red flag when examining the patient. Among these are macular holes, mactel, and vascular diseases, for example, central retinal vein occlusion (CRVO) and central retinal artery occlusion (CRAO). Detecting even one of these pathological conditions in the early stages of their development is crucial for preventing vision loss.
However, many eye care specialists sometimes don’t have enough resources to dedicate more time to analyzing patients’ images. Our recent survey detected that among more than 300 participating optometrists, 40% of them have more than 10 OCT exams per day. Meanwhile, 35% of eye care specialists have 5-10 OCT examinations per day. The greater the number of patients per day, the greater the likelihood that eye care specialists may miss some minor, rare, or early conditions.

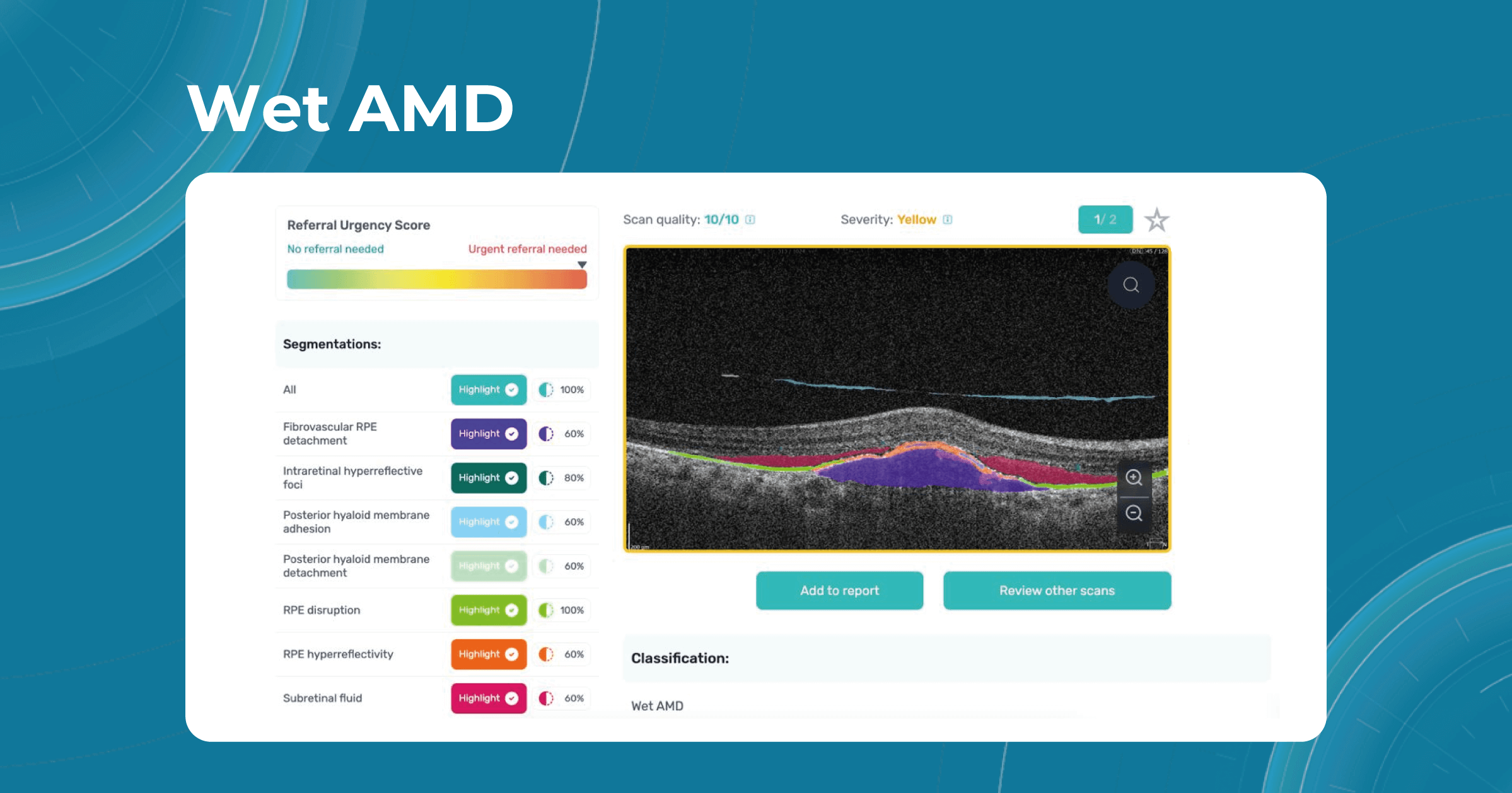
Fortunately, nowadays, there are a lot of ways to empower the clinical workflow, and AI blindness prevention tools are gaining popularity. Artificial intelligence systems like Altris AI can analyze retinal images and other diagnostic data to detect early signs of age-related eye diseases. Altris AI platform, for example, can detect 70+ pathologies and pathological signs, including the ones, that refer to age-related diseases.

Altris AI platform allows eye care specialists to rely on its disease classification when diagnosing a patient. It detects all the most common age-related pathologies, such as AMD, mactel, and vascular diseases – CRVO, CRAO.

Diabetes and diabetic retinopathy
Diabetes and related conditions are also common causes of vision loss. In the United States, about 12% of all new cases of blindness are caused due to diabetes. Globally, diabetes is estimated to cause 4.8% of all blindness. In addition, the risk of blindness from diabetes increases with the duration of diabetes. People with untreated diabetes for years are 25 times more likely to be blind than people without diabetes.

The complication of diabetes, called Diabetic retinopathy (DR), affects the blood vessels of the retina and can lead to impaired vision or blindness. With the rising prevalence of diabetes worldwide, DR has become a significant problem. Early detection, proper control of diabetes, and regular eye exams are essential to prevent vision loss.
The American diabetes association (ADA) recommends that people with diabetes have an OCT scan of their eyes every year. This is because OCT can help to detect early signs of DR with high precision. In some cases, eye care specialists may recommend more frequent OCT scans. This may be the case if the patient has advanced diabetic retinopathy or a family history of diabetic retinopathy.

AI algorithms such as Altris AI can assist in detecting the pathological signs of diabetic retinopathy or diabetic macular edema. Our web platform differentiates certain pathological signs that indicate diabetes-related diseases. Among these are:
- Intraretinal fluid
- Subretinal fluid
- Hard exudates
- Hyperreflective foci
- Epiretinal fibrosis
Genetic and inherited conditions: AI for visually impaired
Some patients are at a greater risk of developing visual impairment due to genetic factors or the inheritance of certain conditions. For example, retinitis pigmentosa is an inherited disease that affects the photoreceptor cells in the retina and gradually leads to night blindness and loss of peripheral vision. Genetic testing and counseling can help identify people at risk and provide early intervention.

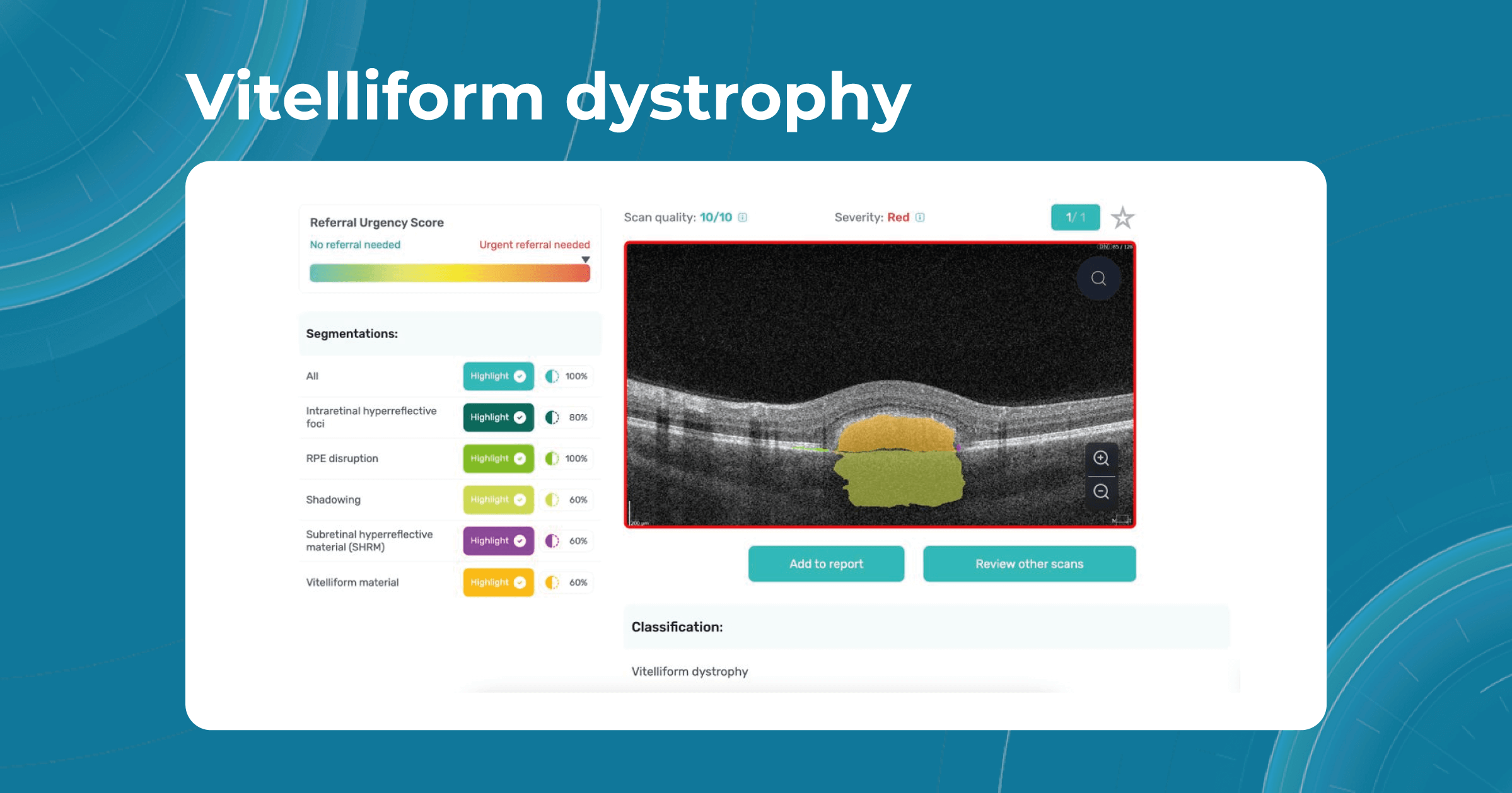
Some genetic eye conditions, such as myopia, vitelliform dystrophy, or retinoschisis, can be detected in the early stages with the help of OCT examination and artificial intelligence systems. Altris AI platform can help eye care specialists in their daily practice and make eye care more accessible, allowing specialists to perform regular eye check-ups, and provide timely treatment of genetic conditions.

Current ways to prevent blindness with AI
As you can see, blindness risk factors encompass a wide range of conditions, pathologies, and circumstances that can significantly impact a patient’s health and increase the likelihood of severe visual impairment. Poorly managed age-related eye diseases, genetic and hereditary factors, and chronic health conditions can lead to eye-related complications, further elevating the risk of blindness.

In the following paragraphs, we will describe in detail the modern ways of using artificial intelligence to detect and prevent blindness: from AI-based retinal imaging for early detection of eye diseases to personalized treatment recommendations and remote patient monitoring.
AI for image interpretation

It is important to understand that the timely detection of eye diseases is key to the effective treatment of visual impairments. However, today we have an unfortunate tendency to diagnose severe forms of disease too late. A large-scale survey by Eyewire conducted in 2021 found that about 40% of people in the USA said they had not had an eye exam in more than a year, and 10% said they had not had one in more than five years.
In addition, recent research by the British Journal of Ophthalmology found that 25.3% of people in Europe over the age of 60 have early signs of AMD. In the UK, about 200 people a day are affected by a severe form of AMD (wet AMD), which can cause severe blindness.
These studies show us that while eye care specialists around the world are trying to treat as many patients as possible, unfortunately, many patients are going blind due to delays in diagnosis. However, using advanced AI-based image analysis systems can speed up the detection of warning signs, allowing you to reach more patients.
One of the advantages of AI for image analysis is its assistance in decision-making. Altris AI is a great example of how an image analysis system can help prevent blindness with AI. The platform allows eye care specialists to detect 74 retina pathologies and pathological signs, including risk conditions for vision loss, like AMD, Diabetic retinopathy, Vascular diseases of the retina, and others.
Diagnosing eye disease in children

Today, one of the most important AI blindness prevention research is focused on teaching artificial intelligence algorithms to detect retinopathy in premature infants. Retinopathy of prematurity is the main cause of childhood blindness in middle-income countries. Some researches show that around 50,000 children all over the world are blind due to the disease.
Unfortunately, experts’ forecasts show that these figures are likely to grow. Retinopathy of prematurity is becoming more and more common, especially in African countries. About 30% of children born in sub-Saharan Africa have this disease and, due to late detection and insufficient attention due to the lack of eye care specialists, can also go blind.
An artificial intelligence model developed by an international team of scientists from the UK, Brazil, Egypt, and the US, with support from leading healthcare institutions, is able to identify children who are at risk of blindness if left untreated. The team of scientists hopes that this AI system will make access to screening and monitoring of young patients more affordable in many regions with limited eye care services and few qualified eye care specialists.
AI monitors for eye strain control
Another interesting application of AI to prevent blindness is eye care monitors. They are planned to be used to avoid eye strain due to prolonged computer work. Such monitors will be programmed to monitor the user’s facial expressions, blinks, and eye movements. They will also be able to assess the level of light in the room, and artificial intelligence will automatically adjust the screen brightness and image contrast.
Since a huge number of the world’s population has switched to remote work since the pandemic and spends almost all day at the computer, such AI monitors are considered a huge help for users in preventing eye diseases that can lead to visual impairment.
AI to determine better glasses or contact lenses

In the field of developing and calculating suitable lenses, there are also a number of companies that have joined the development of AI tools. AI monitors will collect important information about the patient’s eye condition, analyze it, and prescribe suitable contact lenses or glasses.
In addition, these monitors will be able to analyze the patient’s medical history, including medical images, and create the most suitable treatment strategy to maximize visual acuity.
AI for studying the human eye

Today, artificial intelligence for low vision is a promising tool for studying human eye tissue and developing new tools for diagnosing and treating eye diseases, including those that lead to vision loss. Artificial intelligence tools are used to analyze OCT images of the eye to detect changes that may indicate diseases such as diabetic retinopathy, macular degeneration, and glaucoma. AI is also used to predict the development of eye diseases based on genetic or risk factors. This is expected to help doctors identify people at risk of developing eye diseases at an early stage and prevent the progression of the disease.
Summing up
Today AI for eye diseases is already helping eye care professionals, and some companies, like Altris AI, are already using the potential of artificial intelligence to provide early detection and diagnostic advice for eye care specialists. But it’s worth noting that AI tools are not capable of coming up with innovative solutions for blindness prevention.
Only in close cooperation with eye care specialists AI blindness prevention tools can help in many ways, like early detection, providing access to medical care in underserved regions, detecting minor or rare conditions, and allowing to focus on personalized care and treatment of patients.
Disclaimer: USA FDA 510(k) Class II; Altris Image Management System (Altris IMS); AI/ML models and components intended to use for research purposes only, not for clinical diagnosis purposes.