Recently Posted
-

Educating Patients about Eye Health
 Maria Znamenska
26.04.20239 min read
Maria Znamenska
26.04.20239 min readEducating Patients about Eye Health with AI
Today patients are curious about AI, but they may also have some reservations. Researches suggest a cautious attitude towards autonomous AI in healthcare, but what happens when AI becomes a collaborative tool, assisting eye care professionals in educating and treating patients? This shift in focus can significantly affect patients’ comfort levels and acceptance of AI.
Patients have some concerns about AI in healthcare. Let’s delve into the patient perspective and discover how addressing these apprehensions and implementing AI-assisted OCT in eye care can lead to a better understanding of the technology and, ultimately, healthier outcomes.

FDA-cleared AI for OCT analysis
Educating Patients about Eye Health
Interestingly, while surveys extensively document how eye care professionals feel about and interact with AI, the perspectives of the main beneficiary—the patient—remain less understood. The limited research available indicates mixed feelings towards this technology. Few studies examine patient attitudes toward AI in healthcare and eye care, suggesting a degree of caution.

However, these studies have focused on scenarios where AI fully replaces human healthcare providers. Patients demonstrated significant resistance to medical AI in these cases driven mostly by “uniqueness neglect” – concern that AI providers are less able than humans to account for a person’s unique characteristics and circumstances.
For example, in the “Resistance to Medical Artificial Intelligence” study, participants demonstrated less interest in using a stress assessment and were willing to pay less for it when administered by an automated system rather than a human, even with equivalent accuracy. Additionally, participants showed a weaker preference for a provider offering clearly superior performance if it was an AI system.
A survey of 926 patients reveals a mix of attitudes towards AI in healthcare but also gives us clues to understand the reasons behind it. While a majority believe AI could improve care, there’s also a significant undercurrent of caution:
- Desire for Transparency: Over 95% of respondents felt it was either very or somewhat important to know if AI played a significant role in their diagnosis or treatment.
- Unexplainable AI = Uncomfortable: Over 70% expressed discomfort with receiving an accurate diagnosis from an AI system that couldn’t explain its reasoning. This discomfort was more pronounced among those unsure about AI’s overall impact on healthcare.
- Application Matters: Patients were more comfortable with AI for analyzing chest X-rays than for making cancer diagnoses.
- Minority Concerns: Respondents from racial and ethnic minority groups expressed higher levels of concern about potential AI downsides, such as misdiagnosis, privacy breaches, reduced clinician interaction, and increased costs.
These findings highlight the importance of being transparent with patients about how AI is used in their care. Explaining the role of AI and reassuring patients that it’s a tool for assisting your clinical judgment (not replacing it) will be essential. Additionally, being mindful of potential heightened concerns among minority patients is crucial for providing equitable care.
A study solely focused on overcoming patients’ resistance to AI in healthcare found that demonstrating social proof (like highlighting satisfied customer reviews) increased trust in AI-involved help.
The team has identified several additional strategies for reducing patient apprehension of AI recommendations. One effective approach is to emphasize AI’s collaborative nature, where a human doctor endorses recommendations. This highlights AI as a tool to assist, not replace, physicians. Demonstrating AI capabilities through real-world examples where AI exhibits nuanced reasoning can also encourage greater reliance on the technology.
How to attract patients with AI in eye care
AI offers a powerful way to transform your practice and set yourself apart. It brings world-class diagnostic expertise directly to your community, potentially saving patients’ sight by catching eye diseases in their earliest stages. Here’s how to position AI for patients:
- Emphasize Early Detection
It brings world-class diagnostic expertise directly to your community, potentially saving patients’ sight by catching eye diseases in their earliest stages, including early signs of glaucoma, AMD, and many other pathologies that would often be invisible during a regular visit. Some retinal changes are so microscopic that they elude the human eye, making the program’s ability to detect tiny retinal changes invaluable. This makes AI a powerful tool during routine exams, potentially uncovering issues you may not even have been aware of as a patient.
- More time for personalized care with optometry patient education
Patients expect personalized experiences, and AI empowers you to deliver exactly that. By analyzing each patient’s unique OCT image data, AI helps identify potential pathologies with greater accuracy.

Additionally, since AI acts as a meticulous assistant, double-checking your assessments and minimizing the risk of missed diagnoses, it frees up your time. This allows for more meaningful one-on-one conversations with patients, where you can explain their results and discuss the next steps, setting your practice apart regarding patient satisfaction.
- Your old good eye care professional, but with superpower
With AI-assisted OCT, you have the combined knowledge and experience of leading eye care specialists at your fingertips for every patient. This technology leverages massive datasets of medical images and clinical data meticulously analyzed by retinal experts during AI development. It is a valuable second opinion tool, helping you confirm diagnoses and identify subtle patterns the human eye might miss.

This offers your patients peace of mind – knowing their diagnosis has been informed by insights from a team of experts incorporated into the AI’s analysis.
It’s crucial to emphasize that AI will never replace the human touch. It’s a powerful tool that frees up your time for what matters most: building trust through personalized care and addressing patient concerns with empathy.
How to explain what AI is to patients

Patient understanding is vital for building trust with you and any technology you use. It is especially important when talking about a sophisticated instrument like AI. In case of AI, which remains a mystery to many, patient education in optometry is a must.
For instance, we’ve found that patients sometimes struggle to understand how Altris AI, our AI-powered OCT analysis tool, works. We’ve crafted an explanation that helps them grasp the concept more quickly, covering how retinal specialists have taught the system to do its job, the AI’s role as a doctor’s help, and direct benefits for patients.
OCT scans provide incredibly detailed images of the retina, the important layer at the back of your eye. Eye doctors carefully analyze these scans to spot any potential problems. To make this process even more thorough, AI systems are now being used to assist with OCT analysis.

How does the system know how to do that? Real doctors have taught it. It works by first learning from thousands of OCT scans graphically labeled by experienced eye doctors.
The doctors analyzed images from real patients to detect and accurately measure over 70 pathologies and signs of pathology, including age-related macular degeneration and glaucoma, teaching the AI what to look for.
The system leverages a massive dataset of thousands of OCT scans collected from 11 ophthalmic clinics over the years. Carefully segmented and labeled by retinal professionals, these scans were used to train the AI. By analyzing each pixel of an image and its position relative to others, the AI has learned to distinguish between different biomarkers and pathologies.
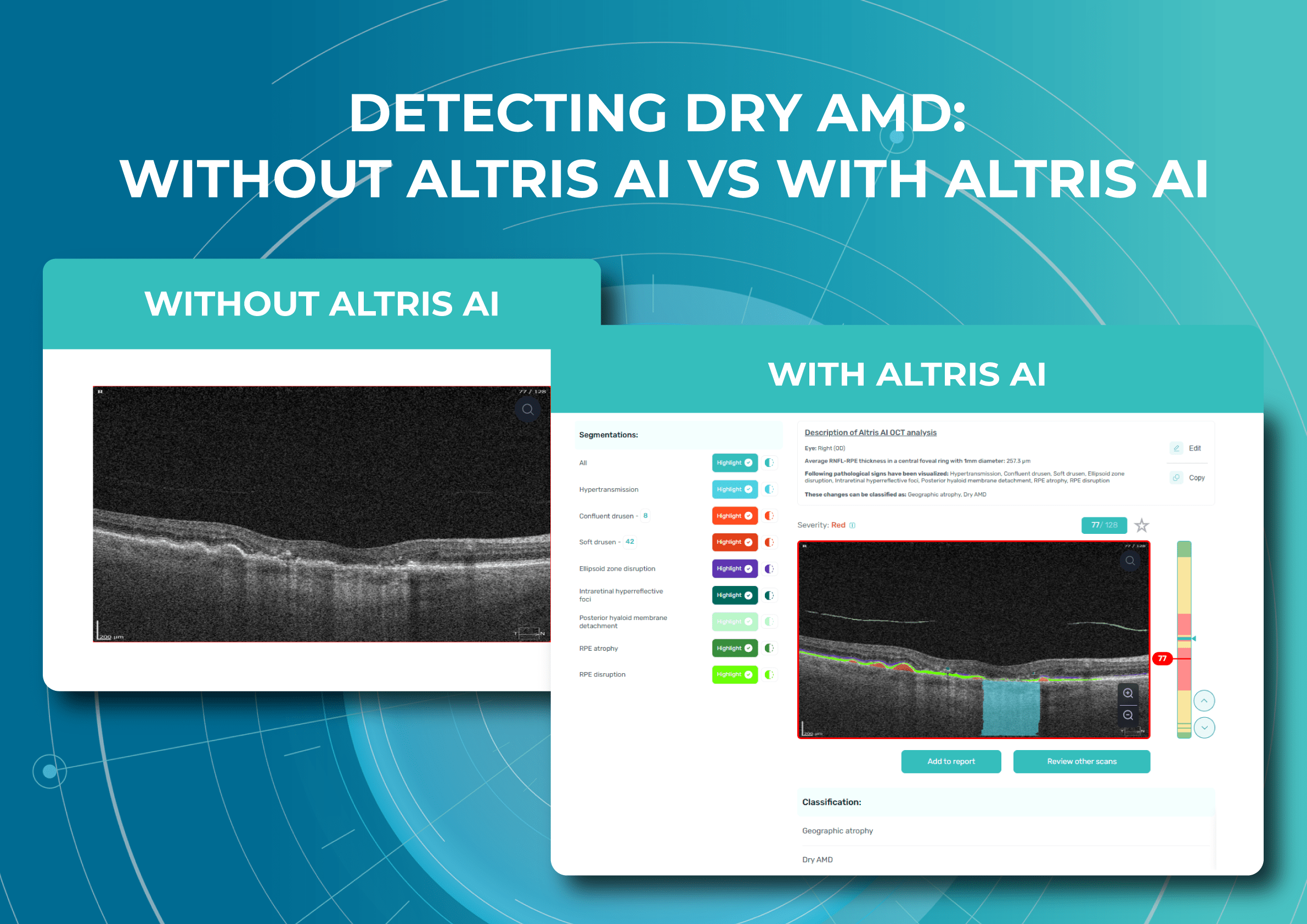
The platform visualizes what is going on with the retina using color coding. This means that every problem on the OCT scan will be colored differently and signed so you will be able to understand what is going on with your retina.

As with any innovative tool, Altris AI partially automates some routine tasks, so clinicians have more time for what is important: talking to patients, learning more about their eye health, and providing treatment advice.
Why does this matter to you? Altris AI can help spot even the tiniest changes in your eyes, leading to earlier treatment and better protection of your eye health. Knowing a smart computer system is also double-checking your scans gives both you and your doctor extra confidence in the results.
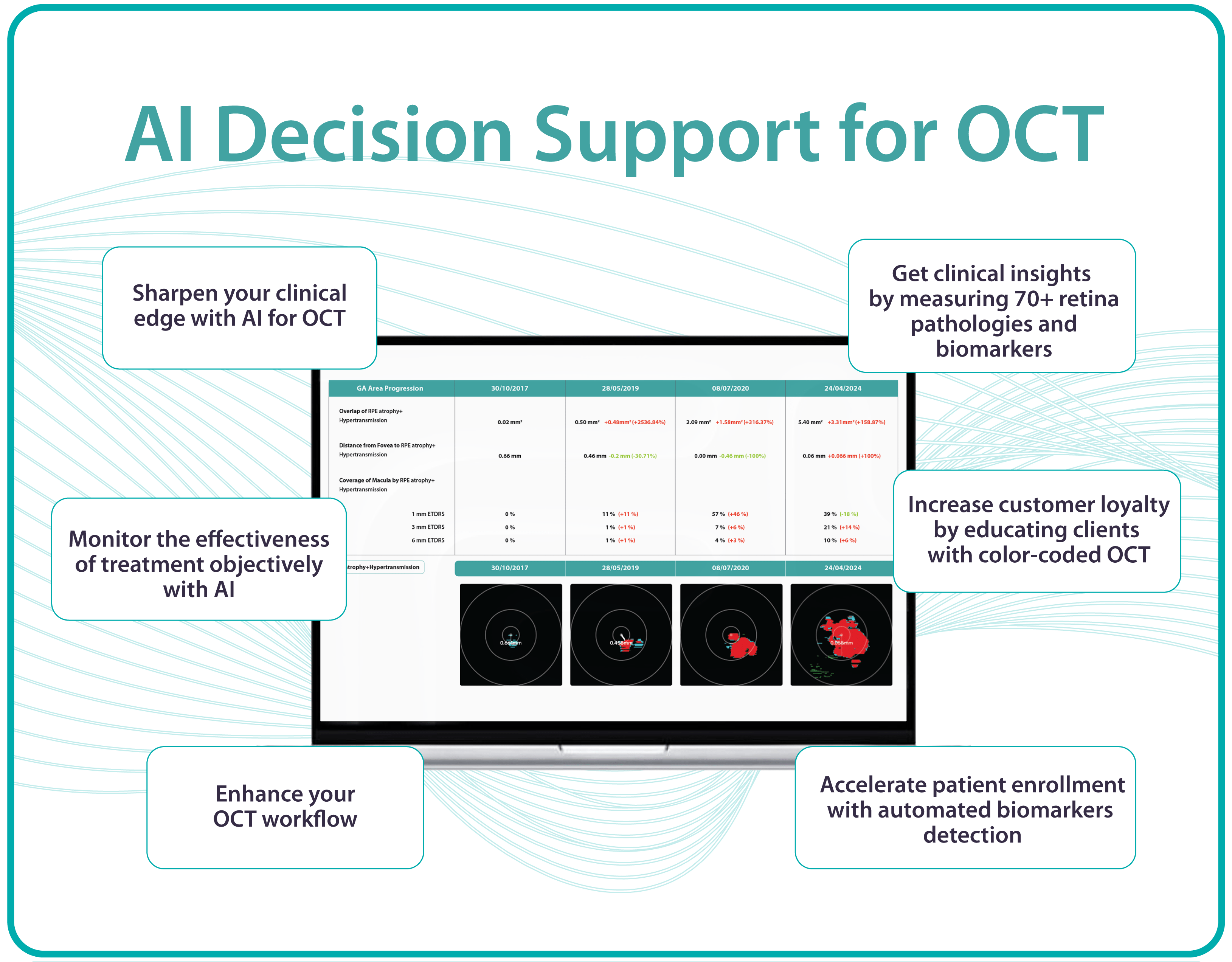
With the help of Altris AI, you will be able to see how the treatment affects you. For example, if you have fluid in the retina (that is not supposed to be there), you will be able to see if its volume is decreasing or increasing with the help of color coding.

Altris AI was designed by eye doctors for eye doctors. It’s a tool to help us take even better care of patients.
AI color coding in eye care: how learning about diagnosis influences treatment adherence
Patient-centered care, a key principle outlined by the Institute of Medicine, emphasizes optometry patient education and involvement in decision-making. This is vital in ophthalmology, where insufficient patient engagement can lead to irreversible blindness.
Research specifically targeting the ophthalmology patient population, which often includes older and potentially visually impaired individuals, reveals a clear preference for individualized education sessions and materials endorsed by their eye care provider.
According to Wolters Kluwer Health, patients crave educational materials from their providers, yet only two-thirds actually get them. This leaves patients searching for information, potentially exposing them to unreliable sources.
Providing clear, accessible patient education is crucial to ensure understanding and treatment adherence.
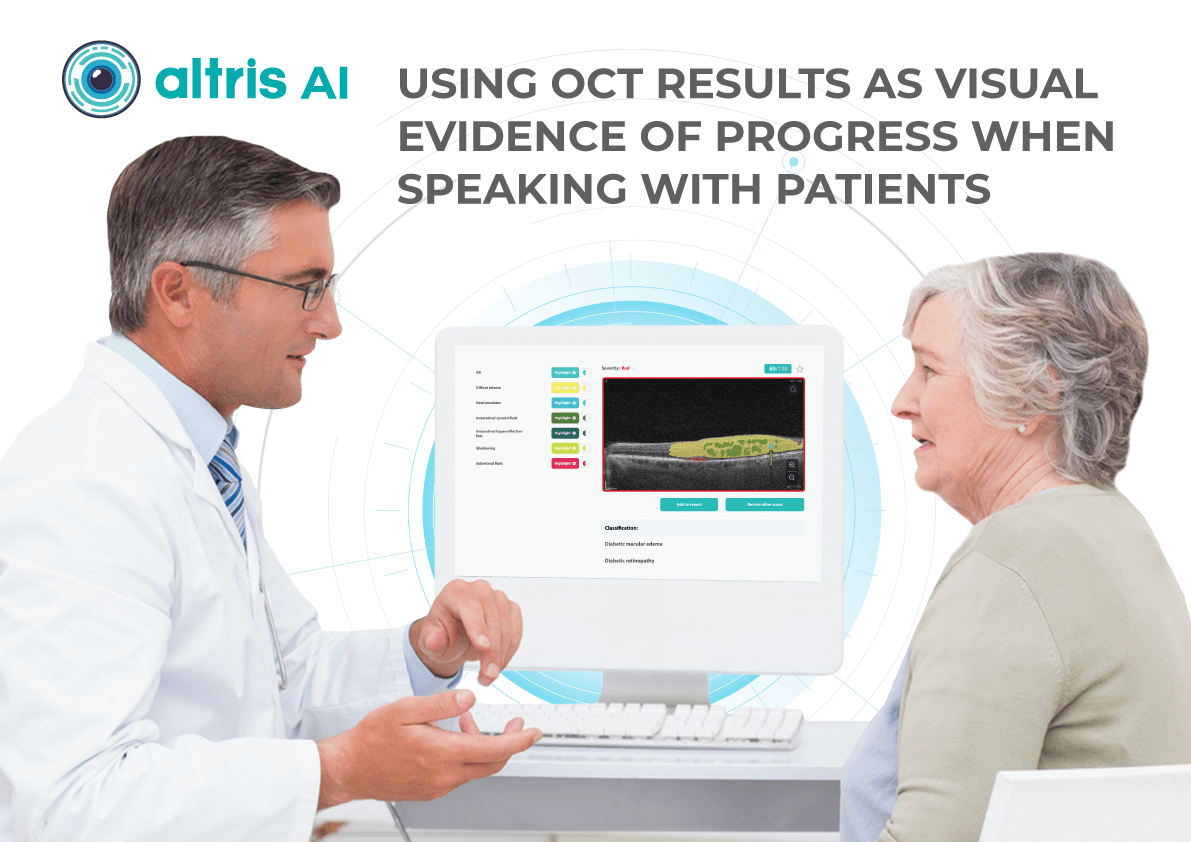
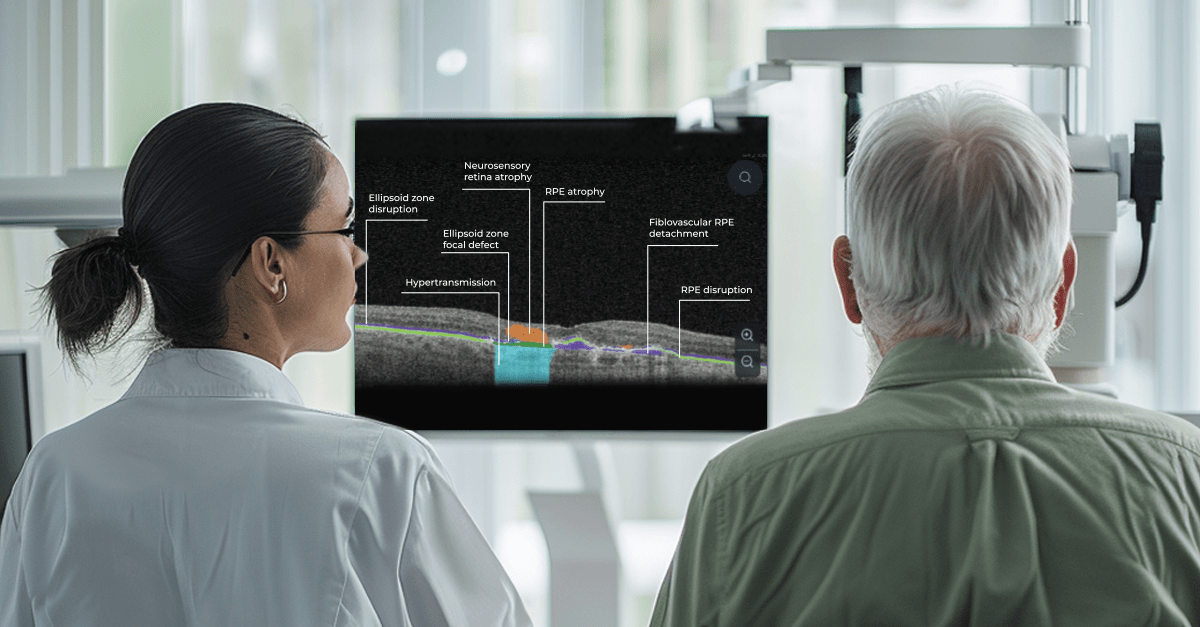
The human brain’s ability to process visual information far surpasses its speed with text, making visual aids a powerful tool for health education. In the field of eye care, this becomes even more critical. Patients often experience vision difficulties, potentially hindering their ability to absorb written materials. Providing clear visual representations of diagnoses can significantly improve patient understanding and compliance.
A study shows a strong preference for personalized educational materials, especially among older visually impaired patients. Seeing photos of their condition, like glaucoma progression, builds trust and reinforces the importance of treatment recommendations.
Surveying eye care professionals specializing in dry eye disease revealed a strong emphasis on visual aids during patient education. Photodocumentation is a favored tool for demonstrating the condition to asymptomatic patients, tracking progress, and highlighting the positive outcomes of treatment.
A visual approach is particularly motivating for patients. It provides tangible evidence of the benefits of their treatment investment, allowing for a deeper understanding of the “why” behind treatment recommendations and paving the way for ongoing collaboration with the patient.
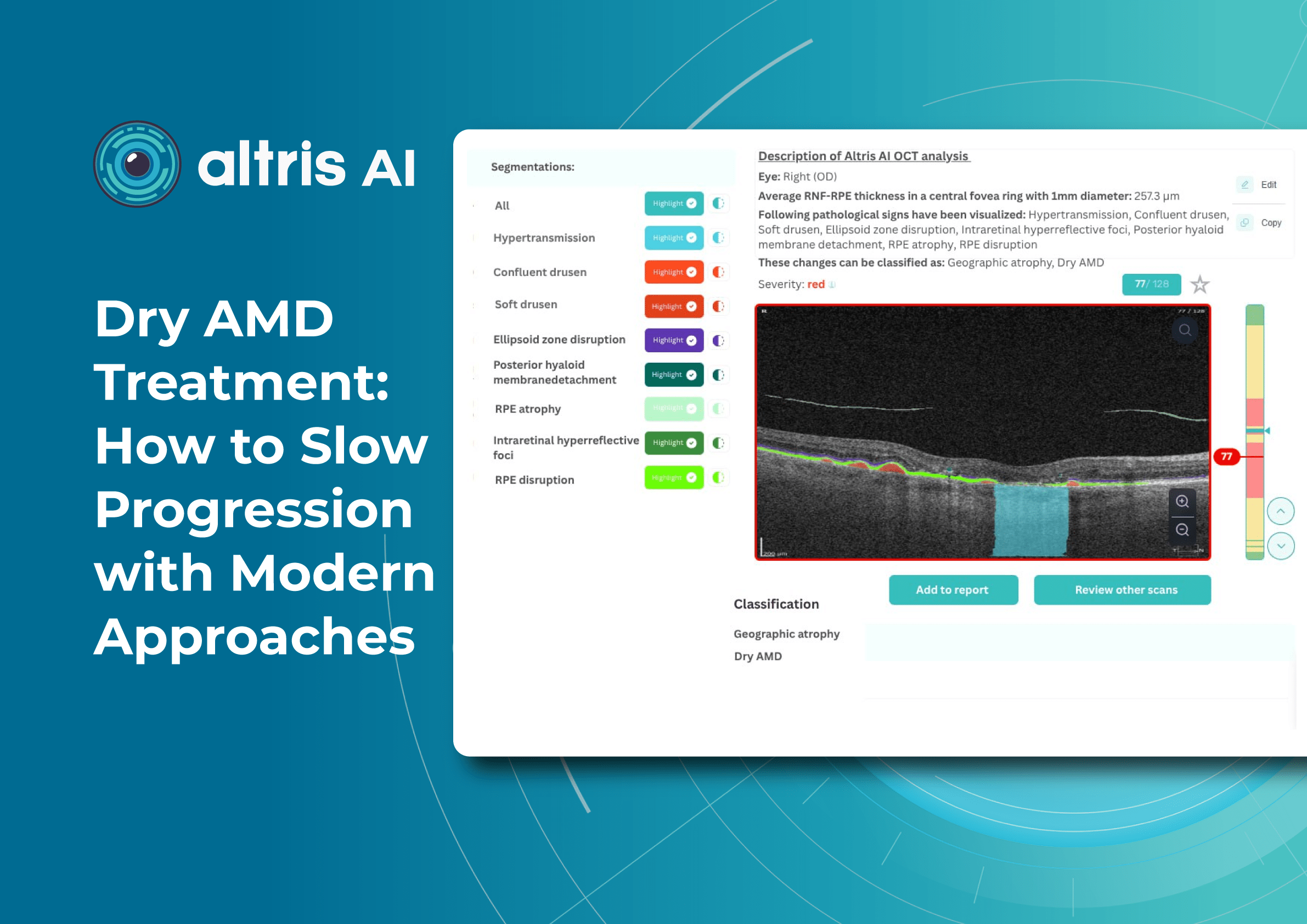
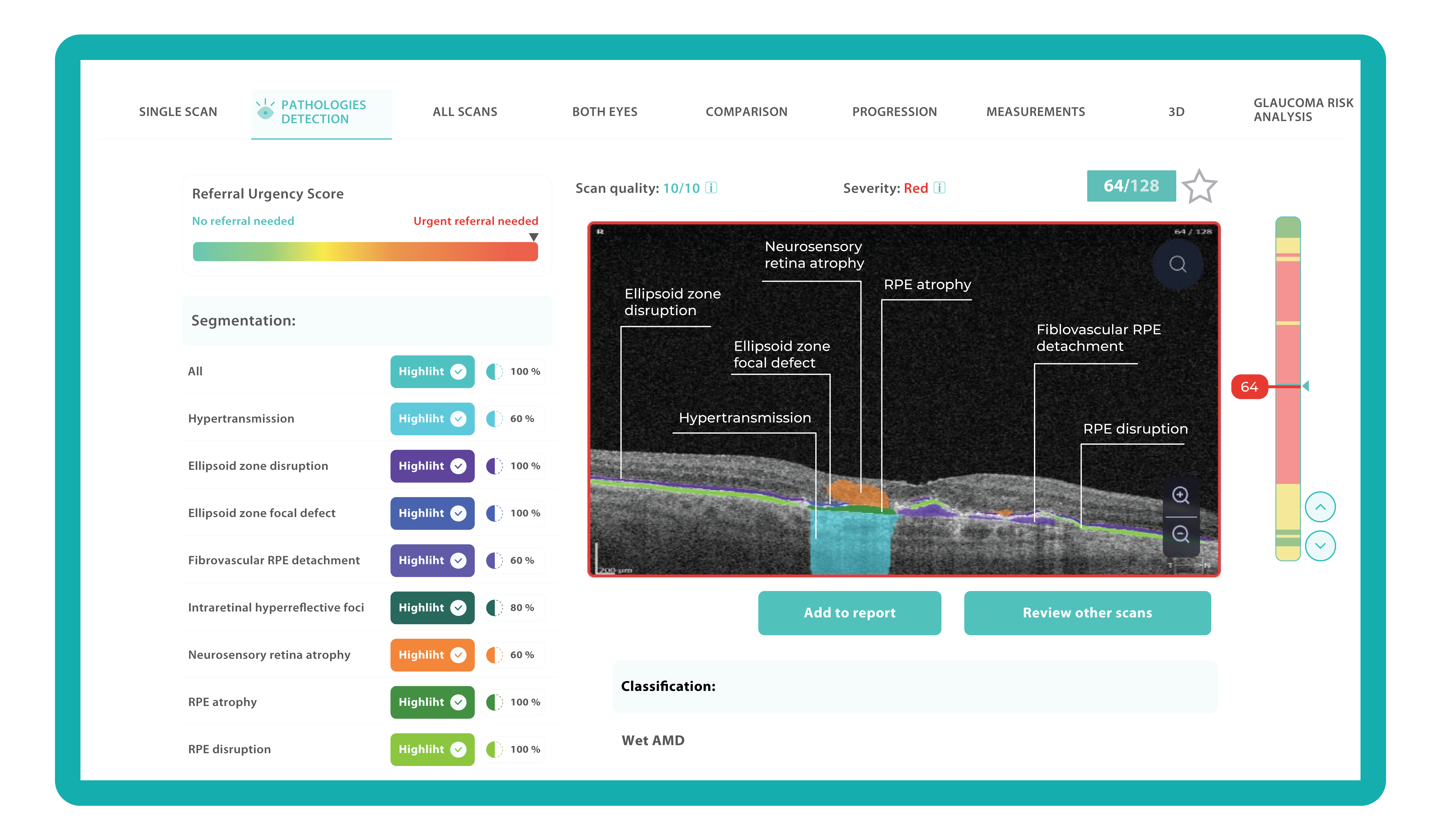
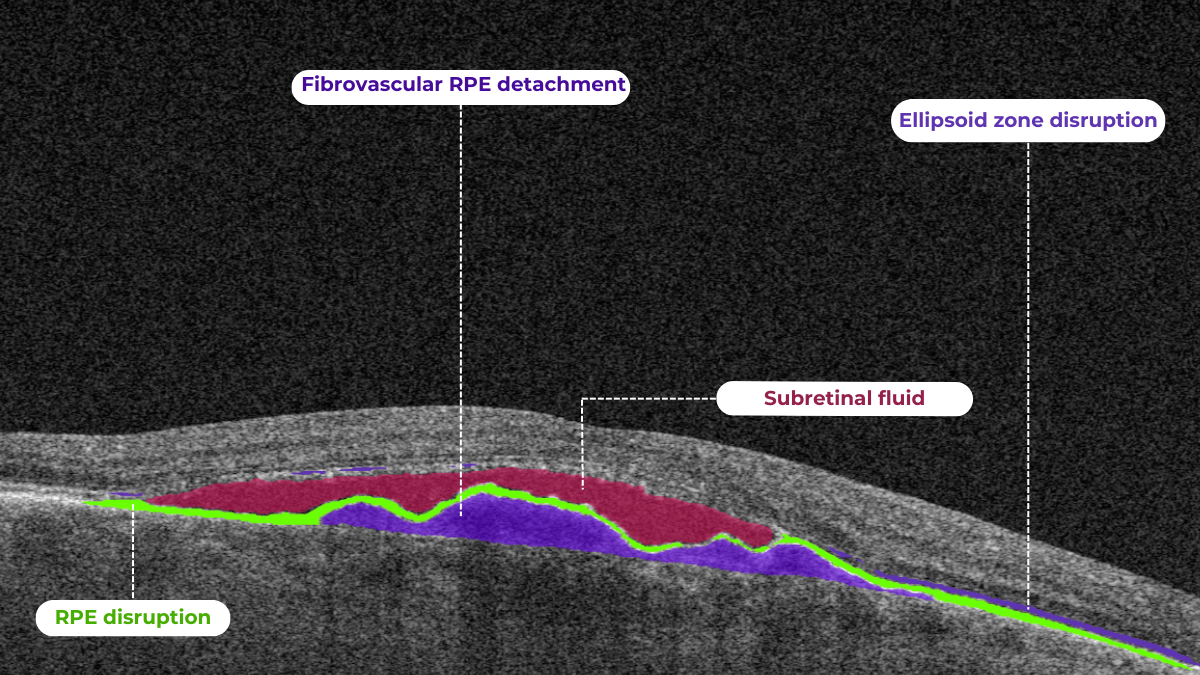
Understanding complex eye conditions can be challenging for patients. Altris AI aims to bridge this gap by using color coding for pathologies and their signs, severity grading, and pathology progression over time within its OCT analysis.
With Altris AI, scans are color-coded for instant interpretation: all the detected pathologies are painted in different colors, highlighting the littlest bits that the unprepared eye of a patient would miss otherwise.

This easy-to-understand visual system empowers patients. They can clearly see what’s happening within their eyes and track the progress of any conditions during treatment.
Eye care professionals are enthusiastic about its impact.

The power of visuals goes beyond understanding a diagnosis. When patients see the interconnected structures that make up their vision, they gain a deeper appreciation for its complexity and the importance of preventative care. This understanding fosters a true partnership between doctor and patient, where the patient is an active, informed participant in their own eye health.
Summing up: Educating Patients about Eye Health

FDA-cleared AI for OCT analysis
Patient education in optometry is vital today and AI is the perfect tool for that. Patients are increasingly curious and open to AI’s potential in general healthcare and eye care in particular, but naturally, some questions and hesitation remain. They stem from a desire to ensure AI considers their individual needs. By addressing these concerns proactively and clarifying when and how AI is used in their care, emphasize the collaborative doctor-AI model—highlight that YOU review and endorse all AI recommendations.
You can successfully integrate this powerful technology into your practice by addressing patient concerns with empathy and highlighting AI’s benefits. This leads to better patient education in optometry and empowered patient experience, improving understanding, adherence to treatment, and, ultimately, better health outcomes.
-

Early Glaucoma Detection Challenges and Solutions
 Maria Martynova
09.04.202310 min read
Maria Martynova
09.04.202310 min readGlaucoma’s silent progression highlights a challenge we all face as clinicians. Millions of individuals remain at risk for irreversible vision loss due to undiagnosed disease – 50% or more of all cases. This emphasizes our responsibility to enhance early detection strategies for this sight-threatening condition.
Existing clinical, structural, and functional tests depend on both baseline exams and the need to observe changes over time, delaying the assessment of treatment effectiveness and the identification of rapid progression.
In this article, we will consolidate our knowledge as eye care professionals about Glaucoma, explore current clinical detection practices, and discuss potential areas to optimize early Glaucoma detection.

FDA-cleared AI-powered OCT Glaucoma Risk Assessment
What we know about Glaucoma
Glaucoma is a complex neurodegeneration fundamentally linked to changes occurring in two locations: the anterior eye (elevated pressure) and the posterior eye (optic neuropathy). Factors influencing glaucoma development include:
- age,
- ethnicity,
- family history,
- corneal thickness,
- blood pressure,
- cerebrospinal fluid pressure,
- intraocular pressure (IOP),
- and vascular dysregulation.
Early stages of Glaucoma are often asymptomatic, highlighting the importance of comprehensive eye exams, even without apparent vision issues. Current diagnostic criteria are insufficient and lack markers of early disease.
Glaucoma is broadly divided into primary and secondary types, with primary open-angle Glaucoma (POAG) representing approximately three-quarters (74%) of all glaucoma cases.
Primary glaucomas develop independently of other eye conditions, while secondary glaucomas arise as a complication of various eye diseases, injuries, or medications.
POAG is characterized by an open iridocorneal angle, IOP usually > 21 mmHg, and optic neuropathy. Risk factors include age (over 50), African ancestry, and elevated IOP. While IOP is a significant factor, it’s unpredictable – some patients with high IOP don’t develop Glaucoma, and some glaucoma progresses even at normal IOP.
Normal-tension Glaucoma (NTG) shares POAG’s optic nerve degeneration but with consistently normal IOP levels (<21mmHg). Vascular dysregulation and low blood pressure are risk factors. While rarer than POAG, IOP lowering can still be beneficial.
Primary Angle-Closure Glaucoma (PACG) is caused by narrowing the iridocorneal angle, blocking aqueous humor flow. More common in East Asian populations, it can be acute (severe symptoms, IOP often > 30mmHg) or chronic.
Secondary glaucomas are caused by underlying conditions that elevate IOP. Examples include pseudoexfoliative, neovascular, pigmentary, and steroid-induced Glaucoma.
Age is a central risk factor for glaucoma progression, linked to cellular senescence, oxidative stress, and reduced resilience in retinal ganglion cells and the trabecular meshwork. Intraocular pressure (IOP) remains the most significant modifiable risk factor. Understanding individual susceptibility to IOP-related damage is crucial. Existing IOP-lowering treatments have limitations in both efficacy and side effects.

Glaucoma has a strong genetic component, with complex interactions between genes, signaling pathways, and environmental stressors. For now, we know that mutations in each of three genes, myocilin (MYOC), optineurin (OPTN), and TANK binding kinase 1 (TBK1), may cause primary open-angle Glaucoma (POAG), which is inherited as a Mendelian trait and is responsible for ~5% of cases (Mendelian genes in primary open-angle Glaucoma).
More extensive effect mutations are rare, and more minor variants are common. Genome-wide association studies (GWAS) reveal additional genes potentially involved in pressure sensitivity, mechanotransduction, and metabolic signaling.
Recent research also suggests a window of potential reversibility even at late stages of apoptosis (a programmed cell death pathway, which is likely the final step in RGC loss). Cells may recover if the harmful stimulus is removed. This offers hope that dysfunctional but not yet dead RGCs could be rescued.
The Challenges of Early Glaucoma Detection
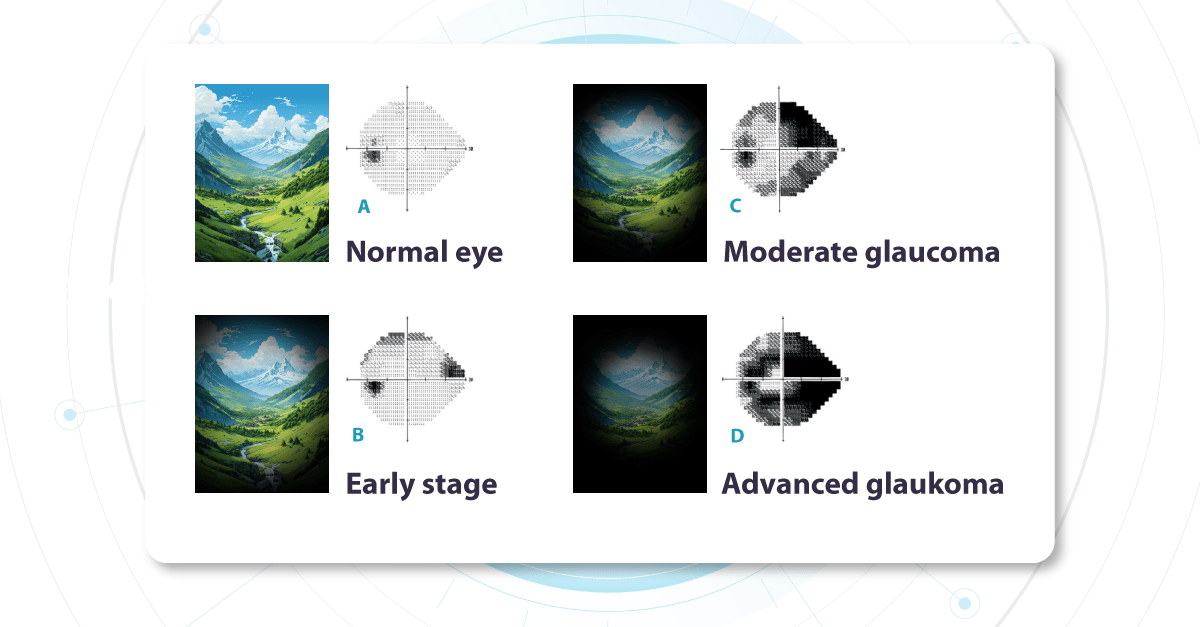
One of the most insidious aspects of Glaucoma is its largely asymptomatic nature, especially in the early stages. This highlights the limitations of relying on symptoms alone and underscores the importance of proactive detection strategies.
Relying on intraocular pressure (IOP) as a stand-alone glaucoma biomarker leads to missed diagnoses, especially in patients with normal-tension Glaucoma. Structural changes, such as optic disc cupping, also lack the desired sensitivity and specificity for early detection.
Optic nerve head evaluations remain subjective, with studies indicating that even experienced ophthalmologists can underestimate or overestimate glaucoma likelihood.
According to the research, even experienced clinicians can have difficulty evaluating the optic disc for Glaucoma. Both trainees and comprehensive ophthalmologists have been found to underestimate glaucoma likelihood in approximately 20% of disc photos. They may also misjudge risk due to factors like variations in cup-to-disc ratio, subtle RNFL atrophy, or disc hemorrhages.
Current Glaucoma Diagnosis in Clinical Practice
Eye care professionals typically encounter new glaucoma diagnoses in one of two ways:
- Firstly, during routine preventive examinations. A patient may come in for various reasons, including work requirements, and be found to have elevated intraocular pressure. This finding prompts further evaluation, potentially leading to a glaucoma diagnosis.
- Secondly, it is a finding in older patients (often over 50-60). A patient may present with significant vision loss in one eye, and examination reveals Glaucoma. Unfortunately, vision loss at this stage is often irreversible.
Alternatively, a patient may seek care for an unrelated eye problem. During the comprehensive examination, the eye care professional may discover changes suggestive of Glaucoma.

As it is statistically prevalent, we most often work with primary Glaucoma, where no other underlying eye diseases are present. Functional changes, specifically as seen on visual field testing, help diagnose and stage glaucoma. During the test, a patient indicates which light signals are visible within their field of vision, building a map of each eye’s visual function.
Vision Field Test for Glaucoma Detection

The optic nerve (a nerve fiber layer of the retina consisting of the axons of the ganglion neurons coursing on the vitreal surface of the retina to the optic disk) transmits visual information from the retina to the brain. Each part of the retina transmits data via a corresponding set of fibers within the optic nerve. Damage to specific nerve fibers results in loss of the associated portion of the visual field.
Challenges with this test include its complexity, especially for older patients, and its subjective nature.
Changes in the visual field determine glaucoma severity. These changes indicate how much of the visual field is already damaged and which parts of the optic nerve are compromised. We call these ‘functional changes‘ as they directly impact visual function.
Fundus photo for Glaucoma detection: What does early glaucoma look like?
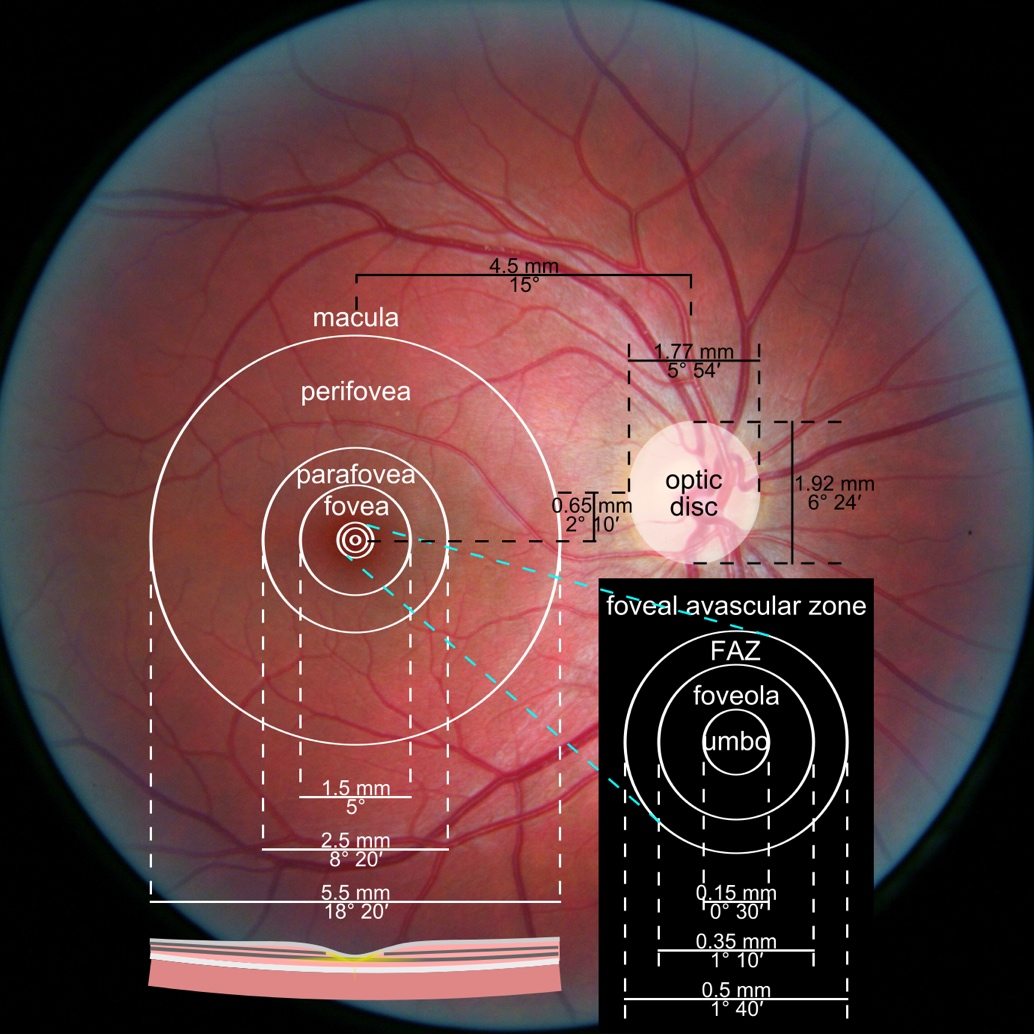
Alongside functional changes, Glaucoma causes visible structural changes in the optic nerve that can be observed during a fundus examination. The optic nerve begins at a point on the retina where all the nerve fibers gather, forming the optic disc (or optic nerve head). The nerve fibers are thickest near the optic disc, creating a depression or ‘hole’ within it. As Glaucoma progresses, this depression deepens due to increased pressure inside the eye. This pressure causes mechanical damage to the nerve fibers, leading to thinning and loss of function.
Another crucial area on the retina is the macula, which contains a high density of receptors responsible for image perception. While the entire retina senses images, the macula provides the sharpest, clearest vision. We use this area for tasks like reading, writing, and looking at fine details. Therefore, the damage to the macular area significantly impacts a patient’s visual quality and clarity. Nerve fibers carrying visual information from this crucial region are essential when evaluating the visual field. We prioritize assessing the macula’s health because it directly determines the quality of a patient’s central vision.
Unfortunately, even if the macula is healthy, damage to the nerve fibers transmitting its signals will still compromise vision.
Glaucoma OCT detection
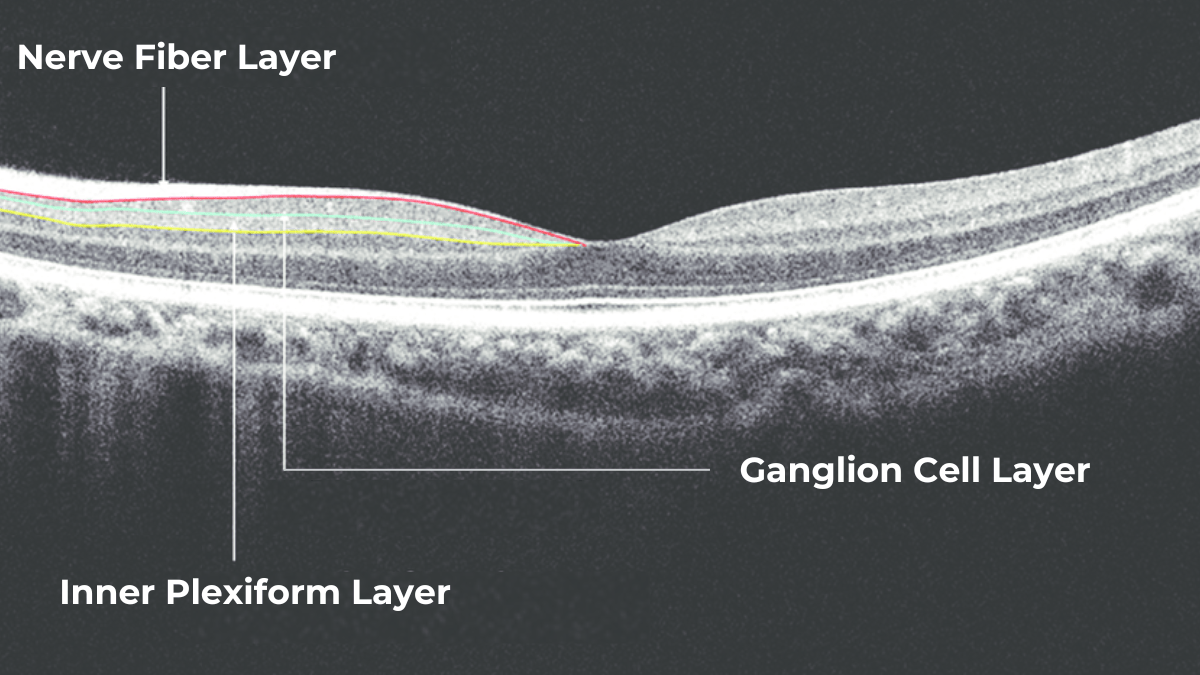
The most effective way to get information about nerve states is OCT, which allows us to penetrate deep into the layers to see the nerve fiber layer separately, making it possible to assess the extent of damage and thinning to this layer in much more detail.

The Glaucoma OCT test provides valuable information about ganglion cells. These cells form the nerve fiber layer and consist of a nucleus and two processes. The short process collects information from other retinal layers, forming the inner plexiform layer. The ganglion cell layer comprises the cell nuclei, while the long processes extend out to create the nerve fiber layer.
Damage to the ganglion cells or their processes leads to thinning across these layers, which we can measure as the thickness of the ganglion cell complex. OCT often detects these microscopic changes before we can see them directly. This enables the detection of structural changes alongside the functional changes observed with standard visual field tests.
Ideally, OCT would be more widely accessible, as the human eye cannot detect early changes. However, how often a patient undergoes OCT depends on various factors. These include the doctor’s proficiency with the technology, the patient’s financial situation (as OCT can be expensive), and the overall clinical picture.
Ways to Enhance Early Glaucoma Detection
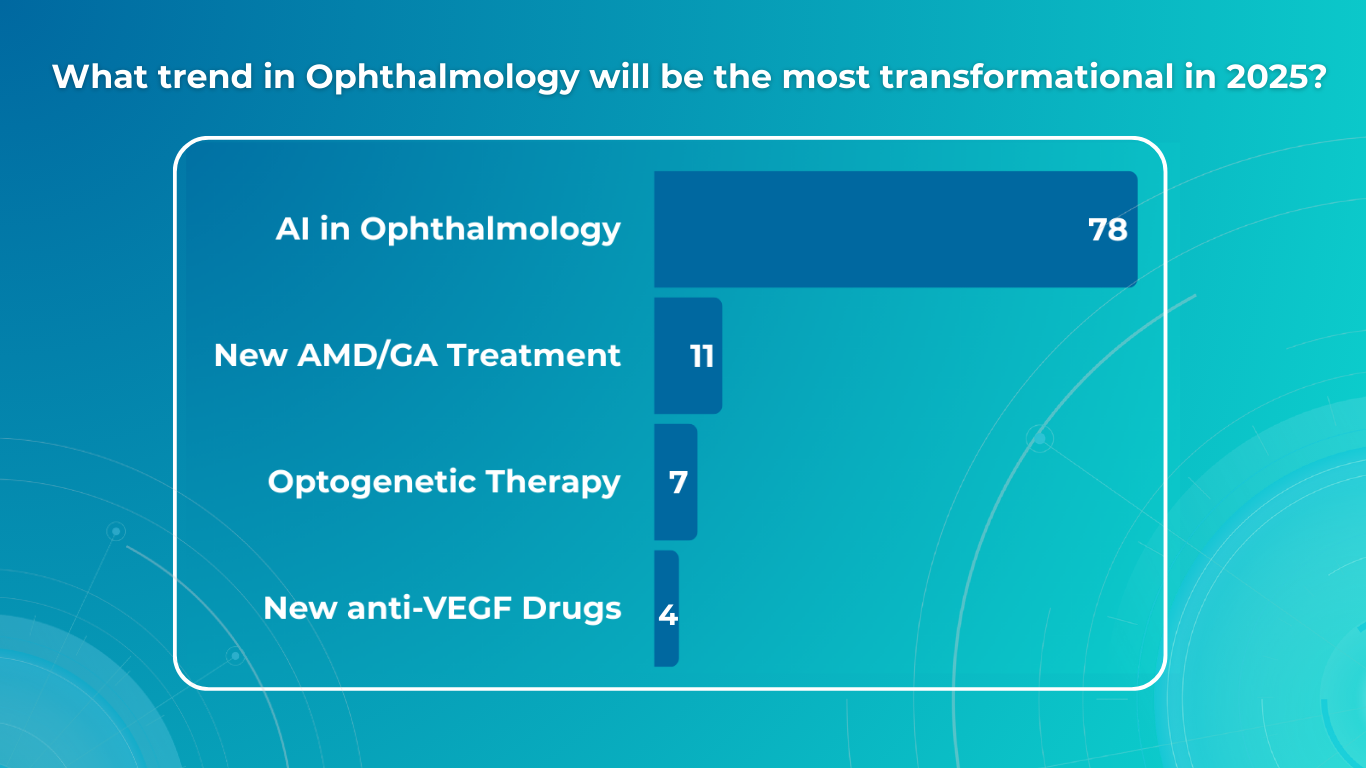
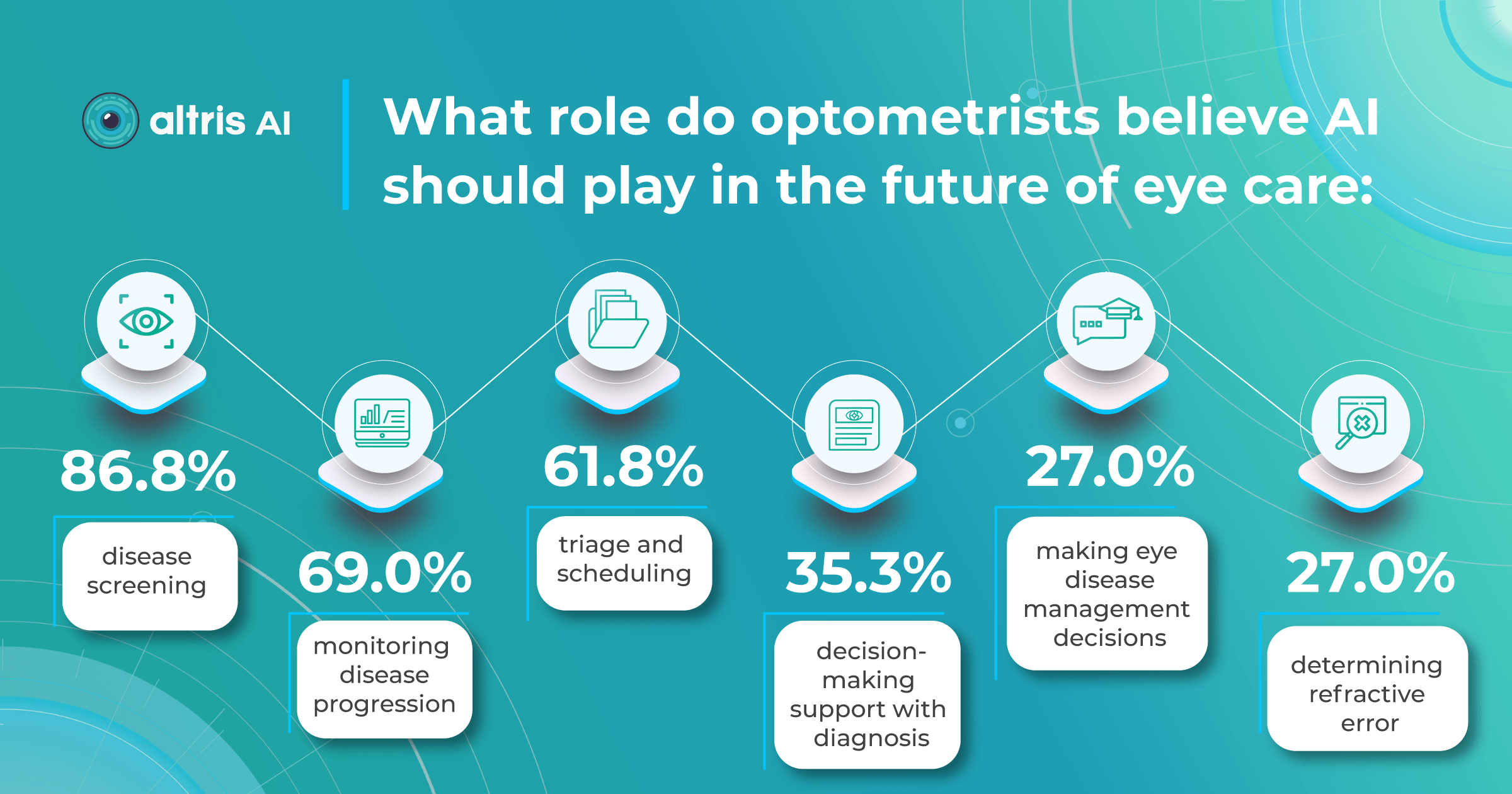
We surveyed eye care specialists, and there was a strong consensus that the most efficient ways to boost early glaucoma detection are regular eye check-ups (47%) and utilizing AI technology (40%). Educating patients was considered less significant (13%).

AI as a second opinion tool
AI offers valuable insights into glaucoma detection, analyzing changes that may not be visible to the naked eye or even on standard OCT imaging.
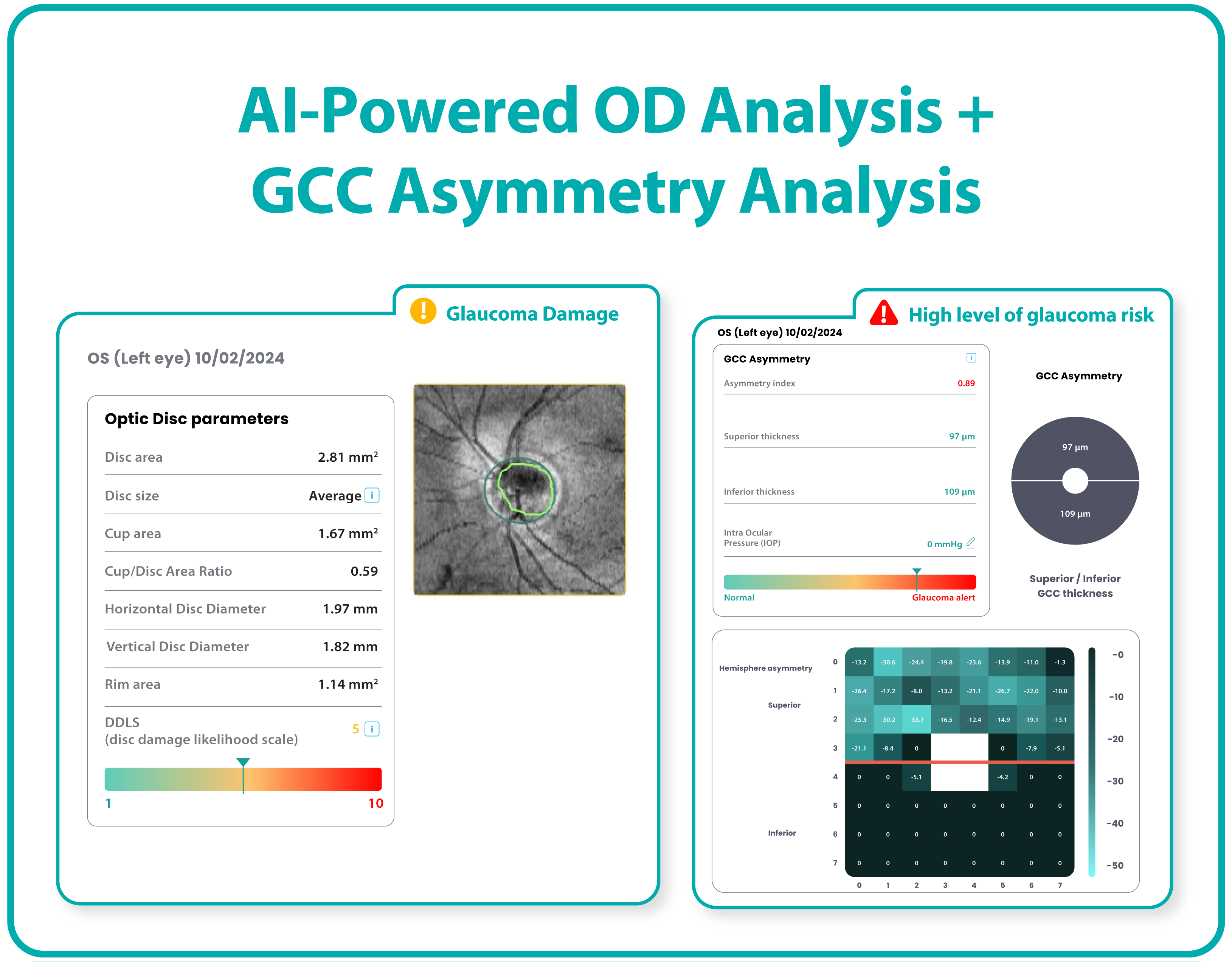
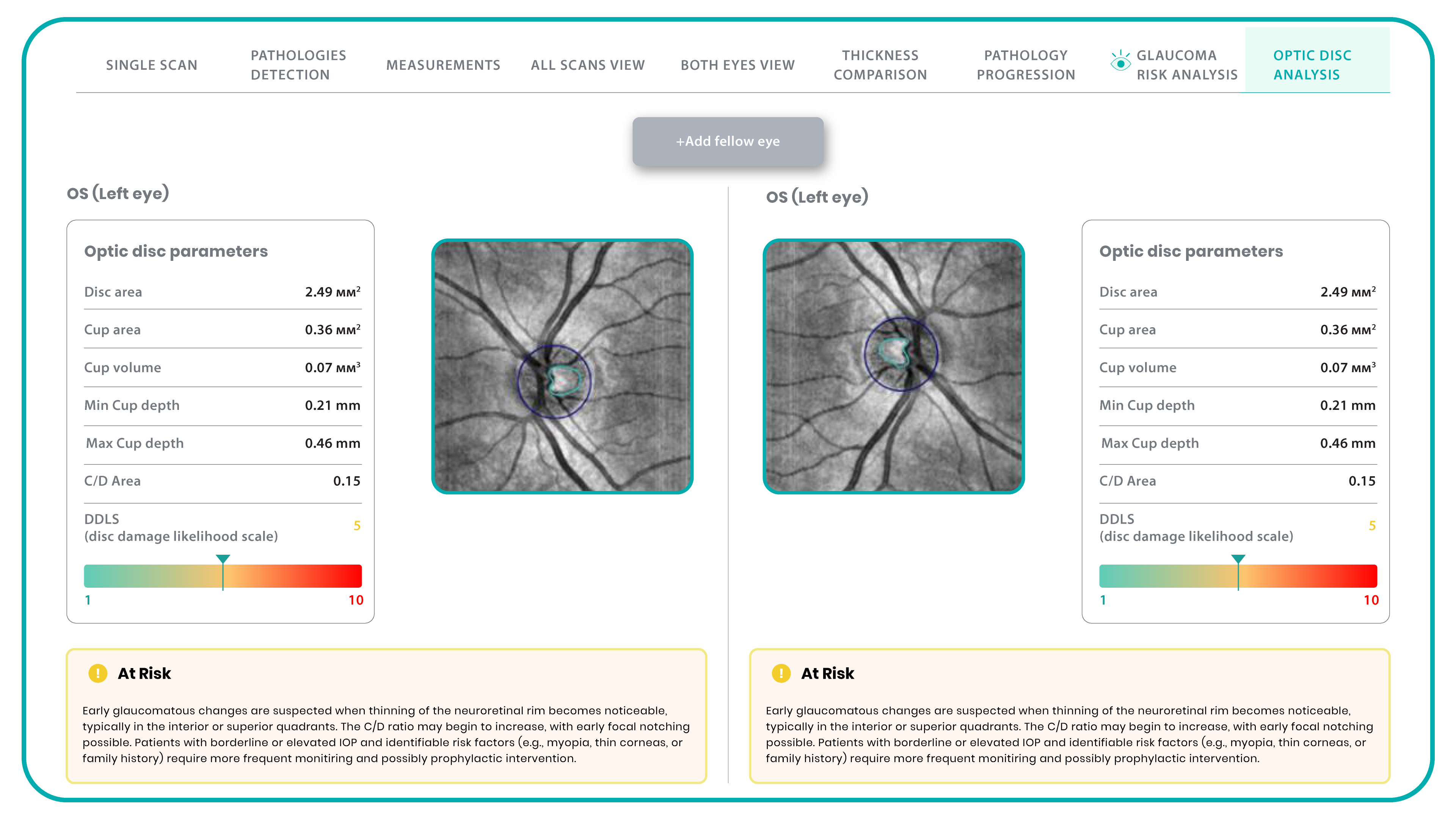
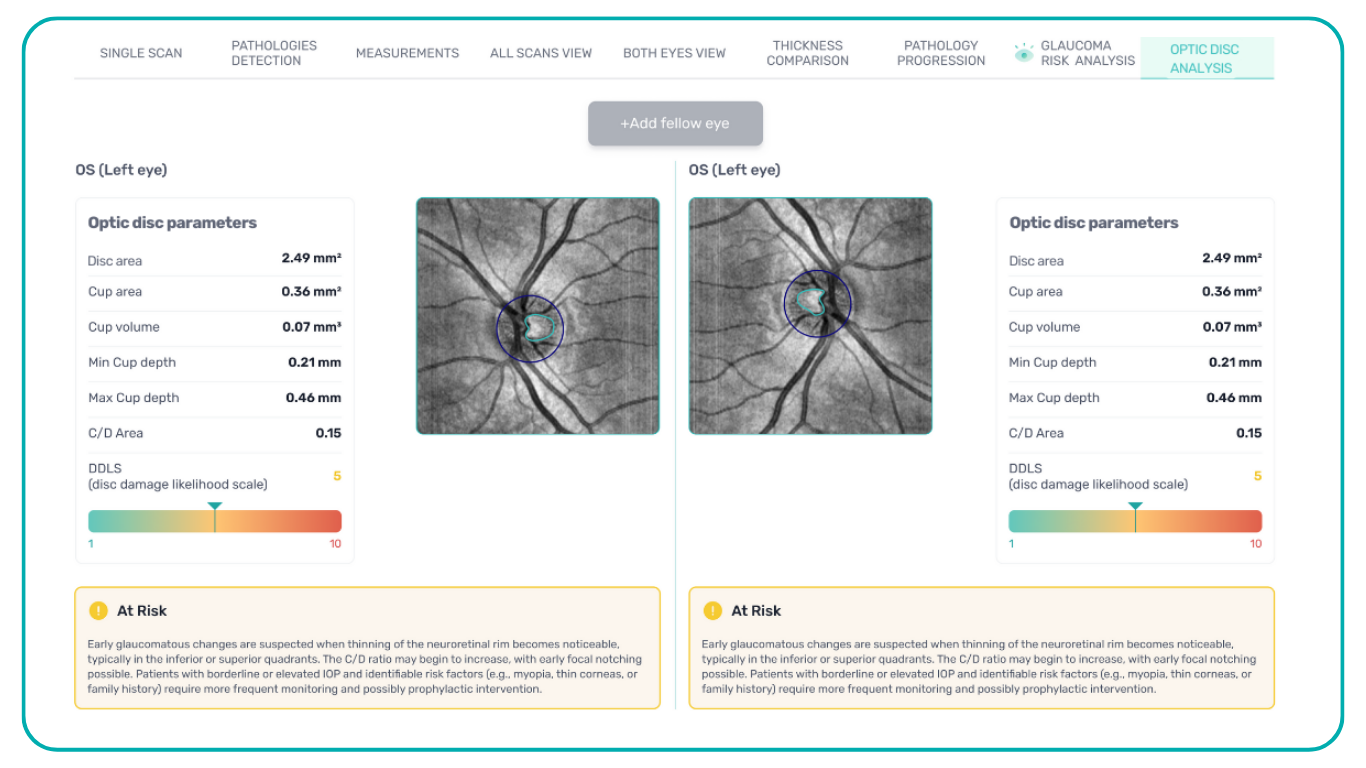
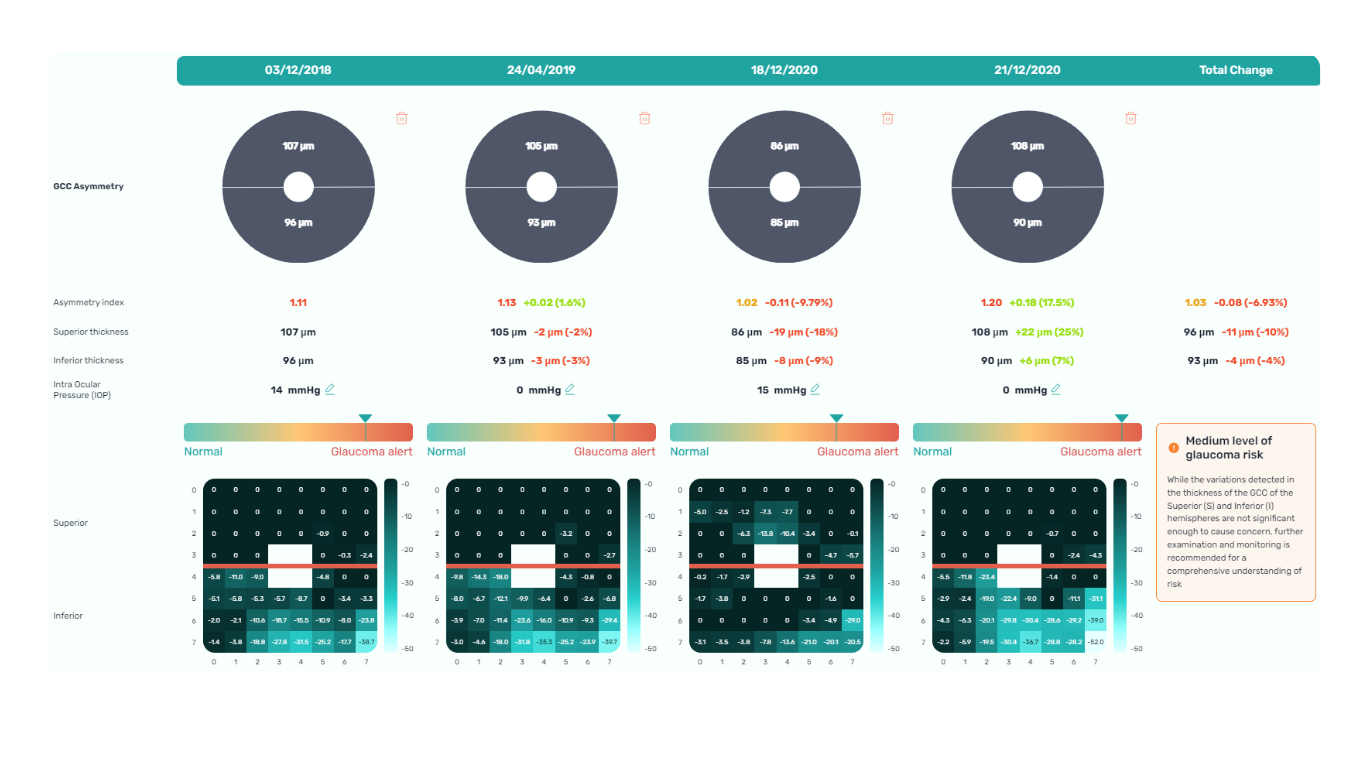
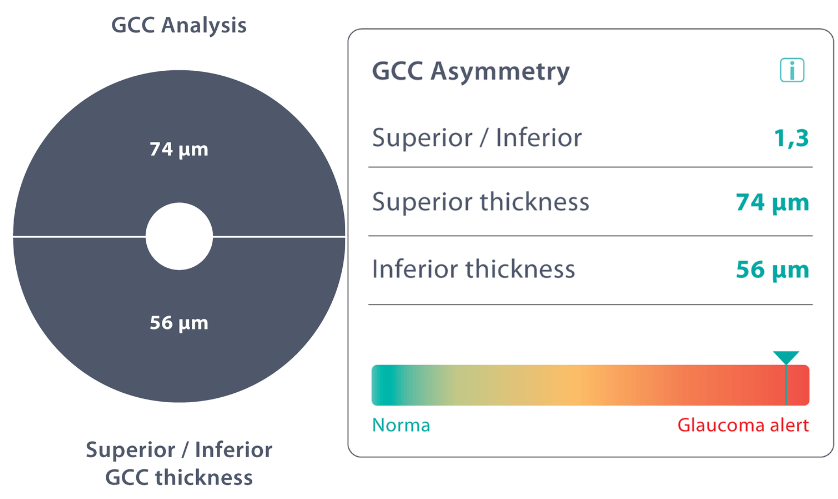
The Altris AI Early Glaucoma Risk Assessment Module specifically focuses on analyzing the OCT ganglion cell layer, measuring its thickness, and identifying any thinning or asymmetry. These measurements help determine a patient’s glaucoma risk. If the ganglion cell complex has an average thickness and is symmetrical throughout the macula, the module will assign a low probability of Glaucoma.

Asymmetries or variations in thickness increase the calculated risk, indicated by a yellow result color. Glaucoma GCC is often characterized by thinning or asymmetry, suggesting glaucomatous atrophy, indicating a high risk, and triggering a red result color.
Changes are labeled as ‘risk’ rather than a diagnosis, as other clinical factors contribute to a confirmed glaucoma diagnosis. Indicators of atrophy could also signal different optic nerve problems, such as those caused by inflammation, trauma, or even conditions within the brain.

It’s crucial to remember that AI ganglion cell layer OCT detection tools like this are assistive – they cannot independently make a diagnosis. Similarly, while helpful in assessing risk, they cannot completely rule out the possibility of developing a disease. This limitation stems from their reliance on a limited set of indicators. Like other technical devices, the module helps flag potential pathology but does not replace the clinician’s judgment.
AI can be incredibly valuable as a supplemental tool, especially during preventive exams or alongside other tests, to catch possible early signs of concern. However, medicine remains a field with inherent variability. While we strive for precise measurements, individual patients, not just statistical averages, must be considered.
Therefore, it is unrealistic to expect devices to provide definitive diagnoses without the context of a complete clinical picture.
Public Health Education

The asymptomatic nature of Glaucoma in its early stages, paired with limited public awareness, creates a fundamental barrier to early detection.
For example, 76% of Swiss survey respondents could not correctly describe Glaucoma or associate it with eye health.
A Canadian study similarly shows that less than a quarter of participants understand eye care professionals’ roles correctly and that most people are unaware eye diseases can be asymptomatic.
Crucially, these studies also found a strong desire across populations for more information about eye care, including Glaucoma (e.g., 97% of Swiss respondents agreed the public lacks knowledge, and 71% want more information). This indicates a receptive audience for targeted education initiatives.
Health education programs, like the USA EQUALITY study, demonstrate the potential to address this challenge. This study combined accessible eye care settings with a culturally sensitive eye health education program, targeting communities with high percentages of individuals at risk for Glaucoma.

Participants showed significant improvements in both glaucoma knowledge (a 62% increase in knowledge questions) and positive attitudes toward the importance of regular eye care (52% improvement).
These results show us that improving glaucoma detection involves more than medical tools. Successful education strategies should prioritize community outreach, partnering with community centers, primary care clinics, and local organizations to reach those lacking access or awareness of regular eye care.
Information about Glaucoma must be presented clearly and accessible, focusing on the basics—what Glaucoma is, its risk factors, and the importance of early detection. Addressing common misconceptions, such as the belief that Glaucoma can’t be present if vision is good, is crucial, as is targeting high-risk groups, including older adults, those with a family history of Glaucoma, and certain ethnicities.
Screening Programs and Regular visits
Community-based studies consistently demonstrate the benefits of targeted screening programs for early glaucoma detection in high-risk populations.
These programs are essential, as traditional glaucoma screening methods often miss individuals with undetected disease.

The USA Centers for Disease Control and Prevention (CDC) funded SIGHT studies focused on underserved communities, including those in urban areas with high poverty rates (MI-SIGHT, Michigan), residents of public housing and senior centers (NYC-SIGHT, New York), and the rural regions with limited access to specialist eye care (AL-SIGHT, Alabama). These programs successfully reached populations who often don’t have regular eye care.
Notably, the results across all three studies demonstrate the effectiveness of targeted programs – approximately 25% of participants screened positive for Glaucoma or suspected Glaucoma.
The SIGHT studies recognize that screening is just the first step, highlighting the importance of follow-up care, testing ways to improve follow-through, using strategies like personalized education, patient navigators, financial incentives, and providing free eyeglasses when needed.
Summing up

FDA-cleared AI-powered OCT Glaucoma Risk Assessment
Glaucoma’s insidious nature demands better early detection strategies. While existing methods are essential, we must also invest in new technologies like AI, enhance public health education about Glaucoma, and focus on targeted screening within at-risk populations. Combining these approaches can protect sight and reduce the burden of glaucoma-related blindness.
-

Effective Eye Care Innovation: Altris AI for the Eye Place
 Altris Inc.
1 min.
Altris Inc.
1 min.The Client: the Eye Place is an optometry center in Ohio, the United States. It is a renowned center that provides comprehensive eye examinations, infant and pediatric eye care, emergency care, LASIK evaluations, and cataract assessment. They offer precise personalized care plans to better treat and prevent ocular disease and chronic illness. Scott Sedlacek, the optometry center owner, is an experienced OD, an American Optometric Association member, and a true innovator who implemented AI for OCT in the optometry practice among the first in the USA.
The Problem: The Eye Place owner has always been searching for innovations to transform the center making it truly digital. The aim of the innovation was also to augment the analysis ability of the optometry specialists using it, while allowing for better visualization of the retinal layers affected for doctors and patients.
The Solution: The Altris AI system was introduced in the Eye Place and it transformed the practice making it more efficient. Scott Sedlacek, the owner of the practice admits that:
“We are one of the first Optometry offices with this AI technology. It is amazing at detecting and defining pathology in the 3D digital images I take with my Topcon Maestro2 OCT. We use Image Net6 software to export Dicom files to Altris AI. It’s fast and easy. If you want the right diagnosis, right away, this is the way to go.
I’ve been using this technology on every patient every day since the beginning of January 2024. There is no other technology in my 25 years being an optometrist that was easier to implement and more impactful immediately.”

FDA-cleared AI for OCT analysis

ROI of the AI for OCT scan analysis
Many eye care specialists worry about the ROI of Altris AI: will the system pay off? After all, it is an investment. That is the experience of Scott, the owner of the Eye Place:
“Altris AI identified and described pathology that I could not. Early detection changes the treatment from doing nothing to something. Also, Altris AI described something that I thought was worse than it was. Saved me from over-referring. Patients love to see the color-coded images which help as an educational tool and get buy-in on the treatment plan which helps compliance. There is a wow factor for me and my patients that sets your practice apart from the others.”
Effective Eye Care Innovation: What Else?
Apart from AI for OCT analysis, the Eye Place utilizes advanced technology for diagnostics.
- For instance, 3D OCT equipment is a highly advanced screening system that checks for serious conditions such as glaucoma, diabetes, macular degeneration, vitreous detachments, and more. Using this technology we can simultaneously take a digital photograph and a 3-D cross-section of the retina.
- Additionally, AdaptDX Pro can detect macular degeneration earlier than by any other means.
- Cognivue Thrive is a personalized, consistent, and reliable way to receive an overall screening of brain health.It is interactive, non-invasive, self-administered, secure, and confidential. It is a five-minute screening for patients of all ages, and you get immediate results in a simple 1-page report.
These are just some examples of innovative tools that optometry centers can use to automate and improve the level of diagnostics. If you want to imagine how Optometry Centers might look like in 2040, here is the article for you. The future is here, and those centers that digitalize have more chances of winning the competition and the hearts of the clients, much like the Eye Place which is highly appreciated by patients.
As you see, effective eye care innovations are an integral part of the work of the Eye Place which is why Artificial Intelligence for OCT analysis was seamlessly integrated into the workflow of the optometry center.
-

Will AI have a Positive Effect on Eye Care Specialists?
 Maria Martynova
18.03.202313 min read
Maria Martynova
18.03.202313 min readVision Care AI: Will AI have a Positive Effect on Eye Care?
Will AI improve your practice or it’s another hype topic that will vanish like NFT or VR glasses?
This article examines present AI’s impact on eye care specialists, exploring its promises and challenges. To gain a realistic view, we surveyed eye care specialists on their experiences and expectations of this topic.
Let’s start with what has already been implemented in eye care and the results we can see already.

FDA-cleared AI for OCT Analysis
Disease screening: DR, AMD, and rare pathologies & biomarkers
A 2022 study by the University of Illinois showed that eye care specialists mostly see AI helping with disease screening, monitoring, and patient triage tasks. Notably, a significant increase in willingness to incorporate AI in practice has emerged after the COVID-19 pandemic, presumably due to a need for remote consultations.

The growing interest in AI for disease screening and monitoring coincides with the development of sophisticated AI systems. Due to their significant causes of visual impairment, Diabetic Retinopathy and AMD are the primary targets for AI screenings.
With over 422 million people worldwide affected by diabetic retinopathy and an estimated 80 million suffering from age-related macular degeneration, the workload on eye care specialists is immense. Unsurprisingly, most AI-powered screening solutions focus on helping clinicians with these diagnoses.
AI algorithms are trained to recognize DR-related alterations on images: hemorrhages, exudates, and neovascularization. AI also offers significant advancements in Age-related Macular Degeneration screening. Algorithms accurately segment data in OCT scans, helping assess retinal structures and quantify fluids during treatment. Trained models predict disease progression risks and analyze treatment responses.
 AI in eye care can segment retinal structures to distinguish between normal retina scans and pathology on OCT, detect atrophic changes, and follow all alterations over time. It can even highlight rare inherited retinal dystrophies. For example, Altris AI is trained to recognize Vitelliform dystrophy and Macular telangiectasia type 2.
AI in eye care can segment retinal structures to distinguish between normal retina scans and pathology on OCT, detect atrophic changes, and follow all alterations over time. It can even highlight rare inherited retinal dystrophies. For example, Altris AI is trained to recognize Vitelliform dystrophy and Macular telangiectasia type 2.Vision Care AI: More Efficient Patient Triage
The number of eye scans clinicians are performing is growing at a pace much faster than human experts are able to interpret them. This delays the diagnosis and treatment of sight-threatening diseases, sometimes with devastating results for patients.
Our recent survey showed that among more than 1000 participating eye care specialists, 40% have more than 10 OCT exams daily. Meanwhile, 35% of eye care specialists have 5-10 OCT daily examinations. Unfortunately, more patients per day mean an increased risk that specialists may miss some minor, rare, or early conditions.

AI systems can quickly triage scans based on severity. Prioritized urgent cases can be flagged for immediate attention. Healthy patients can be monitored without urgency.
This ensures patients with time-sensitive conditions get the care they need, while less urgent cases receive a timely but less immediate review.
Optometrists can use AI for vision care systems to specify the need to refer patients based on eye image analysis.

Another advantage of AI used as a “copilot” is its continuous improvement. Providers that create such systems usually integrate new data and research findings into algorithms, resulting in an ever-evolving resource for eye care specialists.
In other words, the accuracy of the patients’ triage will get better and better with the data.
Early Glaucoma Detection: AI for Vision Care that Works
Glaucoma is a leading cause of vision-related morbidity worldwide. Although blindness is the most feared outcome, even mild visual field loss may harm the quality of life.
In a way, glaucoma is one of the most challenging eye diseases that specialists must treat; with most eye problems, the patient comes when something is wrong. Glaucoma, however, has no symptoms until it is advanced, and the damage can not be reversed.
One common reason glaucoma is not diagnosed early is the inability to recognize glaucomatous optic disc and RNFL damage. Ophthalmologists often rely primarily on intraocular pressure and visual fields and not on the appearance of the optic disc.
Combining optical coherence tomography imaging and artificial intelligence, Altris AI offers a solution to the problem. The platform performs Ganglion Cell Complex asymmetry analysis on OCT scan that categorizes the risk of developing glaucoma. Glaucoma Early Risk Assessment Module can help decrease the number of false-positive referrals and increase the standard of care by supporting early diagnosis to improve patients’ prognosis.
Better Education for Patients
Eye care specialists don’t always have time to explain to patients what is going on with their eye health.
Artificial intelligence can easily perform this task. AI systems will also enhance eye care education, offering innovative and immersive learning experiences: with the help of color-coding, user-friendly reports, and chat bots.
AI-generated OCT reports can propel patient education and engagement. By translating complex medical data into clear, visual formats, AI can help understand patients’ diagnoses, significantly improving treatment adherence and fostering greater patient loyalty.
For example, Altris AI employs smart reports with color-coded segmentation of pathologies that are easy for clinicians and their patients to understand.

When patients fully grasp the nature of their eye conditions and track therapy progress, they are far more likely to prioritize annual checkups and actively engage in their care.
Teleoptometry and teleophthalmology
The COVID-19 pandemic has accelerated the adoption of telemedicine, especially in the image-rich field of ophthalmology.
In recent years, many digital home measurement tests have been introduced. These include home-based and smartphone/tablet-based devices, which are cost-effective in specific patient cohorts.
One example is an artificial intelligence-enabled program for monitoring neovascular Age-related Macular Degeneration (nAMD) that uses a home-based OCT device. Patient self-measurements from home have proved to be a valuable adjunct to teleophthalmology. In addition to reducing the need for clinical visits, they serve as a collection of high-quality personal data that can guide targeted management.
Currently, most commercial providers of telemedical services and devices use artificial intelligence. However, these services are not autonomous. AI works simultaneously with so-called “backup” ophthalmologists. If a finding is unknown or unclear to the artificial intelligence, an ophthalmologist reads the image.
Non-medical AI: General Workflow Enhancements
COVID-19 made it crystal clear that healthcare worldwide has a full spectrum of problems, such as staffing shortages, fragmented technologies, and administrative complexities. So, the AI for vision care boom three years after the pandemic has come timely and handy.

Intelligent algorithms can solve the mentioned issues. For example, generative AI can enable easier document creation by digesting all types of reports and streamlining them. It can also ease the administrative workload for short-staffed clinicians (the average US nurse spends 25% of their work time on regulatory and administrative activities).
Probabilistic matching of data across different databases, typical for Machine Learning, is another technology that can take a burden off staff about claims and payment administration.
Patient engagement and adherence also can benefit from the technology. Providers and hospitals often use their expertise to develop a plan to improve a patient’s health, but that frequently doesn’t matter as the patient fails to make the behavioural adjustment. AI-based capabilities can personalize and contextualize care, using machine learning for nuanced interventions. It can be messaging alerts and targeted content that provokes actions at needed moments or better-designed ‘choice architecture’ in healthcare apps.
Another side of the coin: AI for OCT limitations
When discussing AI in eye care, it’s essential to recognize that AI is a tool. Like any tool, it is neutral. So, its effectiveness and potential for unintended consequences hinge not only on the quality of its design and the data used to train it but also on the expertise of the healthcare professionals interpreting its output. Here are some of the challenges to keep in mind when working with AI.
AI is fundamentally limited by the datasets used for training. An outsized amount of images can slow training and lead to overfitting, while a lack of demographic diversity compromises accuracy.

One challenge facing AI implementation in medicine is the interdisciplinary gap between technological development and clinical expertise. These fields are developing separately and usually do not intersect. Therefore, cross-collaboration can suffer because tech experts may not understand medical needs, and clinicians may not have the technical knowledge to guide AI development effectively.
So, a successful AI solution requires bridging this breach to ensure AI solutions are grounded in medical realities and address the specific needs of clinicians (Clinical & Experimental Ophthalmology, 2019).
The commercialization of AI will also pose future issues. Trained models will likely be sold with and for implementation with certain medical technologies. Additionally, if AI does improve medical care, it will be essential to pass those improvements on to those who cannot afford them.
Overreliance on the technology can also be a problem.

AI is a tool, like any other equipment in the clinical environment. Decision-making is always on the side of an eye care practitioner who has to take into account many additional data: clinical history, other lab results, and concomitant diseases in order to make a final diagnosis.
And, of course, there are ethical dilemmas. Many practical problems can be solved relatively easily – secure storage, anonymization, and data encryption to protect patient privacy. However, some of them need a whole new field of law. The regulations surrounding who holds responsibility in case of a misdiagnosis by AI is still a significant question mark. Since most current AI algorithms diagnose not so many diseases, there is room for error by omission, and a correct AI diagnosis is not a comprehensive clinical workup.
Summing up

While AI in eye care isn’t without limitations and ethical considerations, its revolutionizing potential is hardly deniable. It already has proven itself working with disease screening, monitoring, and triaging, saving specialists time and improving patient outcomes. AI offers a “second opinion” for complex cases and expands access through telemedicine.

FDA-cleared AI for OCT Analysis
Yet, despite all its promises, the implementation of AI in practice should be seen as a new tool and technique, like the invention of the ophthalmoscope, IOL, OCT, and fundus camera. Optometrists and ophthalmologists will need to combine the best of their clinical skills and AI tools for best practices. Being an innovative tool does not make AI a magic wand, fortunately or not.
-

Technologies in Optometry: Clare and Illingwort & Altris AI
 Altris Inc.
3 min.3 min.
Altris Inc.
3 min.3 min.The Client: Clare and Illingworth, renowned leaders in the field of optometry located in the UK.
The problem: The need to speed up the process of OCT interpretation and unburden the optometry team.
The Solution: Clare and Illingworth have embraced cutting-edge technology to enhance their Optical Coherence Tomography (OCT) analysis workflow. The introduction of Altris AI at this optometry center marks a significant milestone in their commitment to providing high-quality services to patients.
According to one of the owners of the optometry center, Richard, “We are adding a new OCT to one of our practices and will benefit from some extra support with AI to speed up the interpretation of results and assist the busy Optometry team.”
Altris AI, a leading provider of artificial intelligence solutions for healthcare, specializes in developing algorithms and software applications that augment medical imaging analysis. The integration of Altris AI into the British Optometry Center’s OCT workflow brings forth a host of advantages, revolutionizing the way eye conditions are diagnosed and managed.

FDA-cleared AI for OCT Analysis
Try it yourself in our Demo Account or get a Brochure
Technologies in Optometry and Ophthalmology: How AI Helps
One of the key benefits of Altris AI is its ability to automate and expedite the analysis of OCT scans. Traditionally, optometrists spent considerable time manually reviewing and interpreting OCT images.
FDA-cleared Altris AI is created to make the OCT workflow more effective
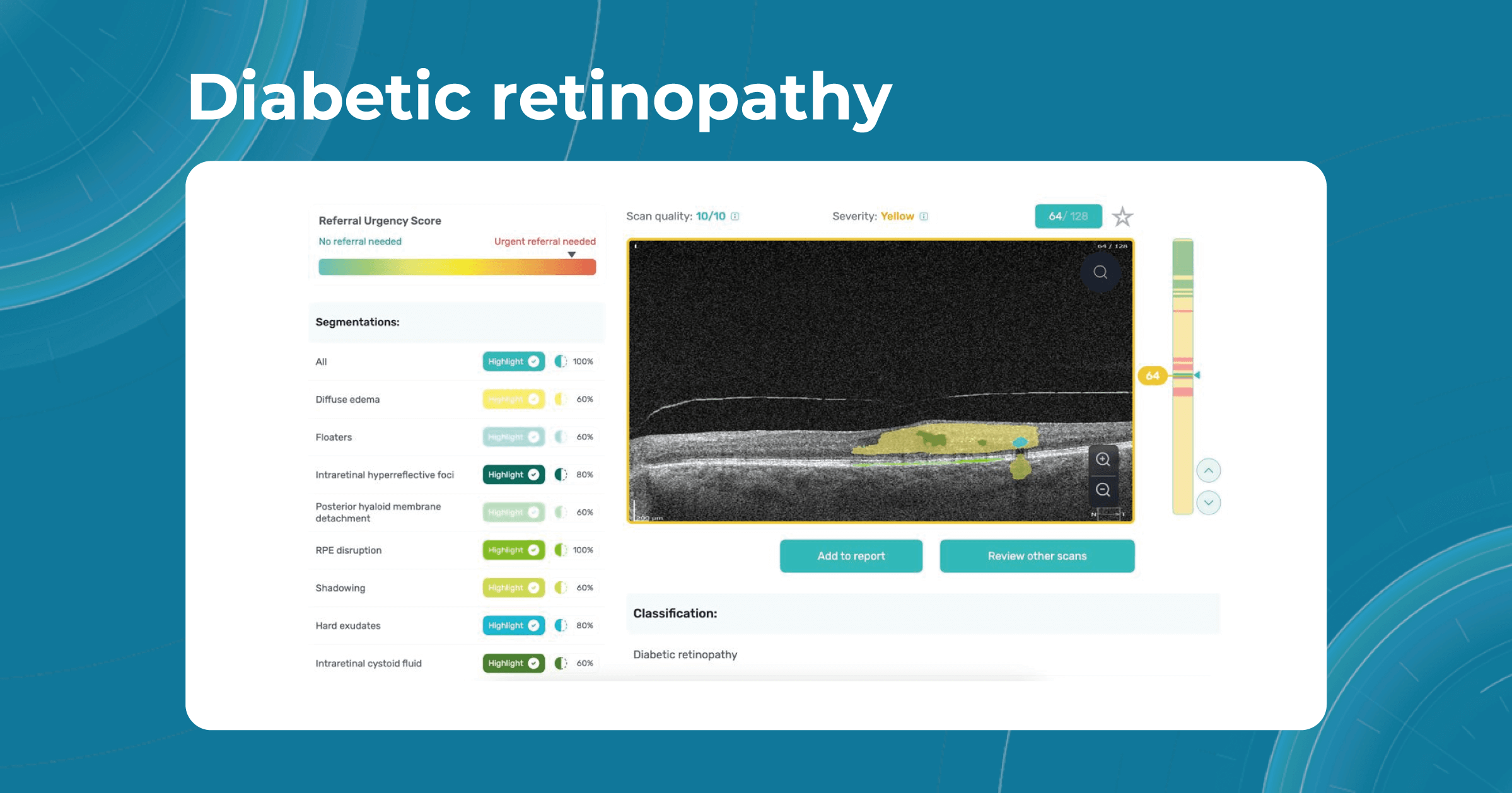
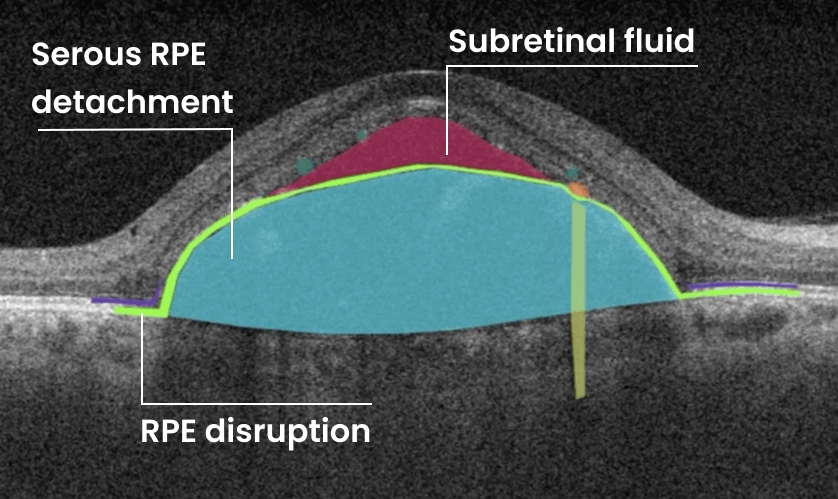
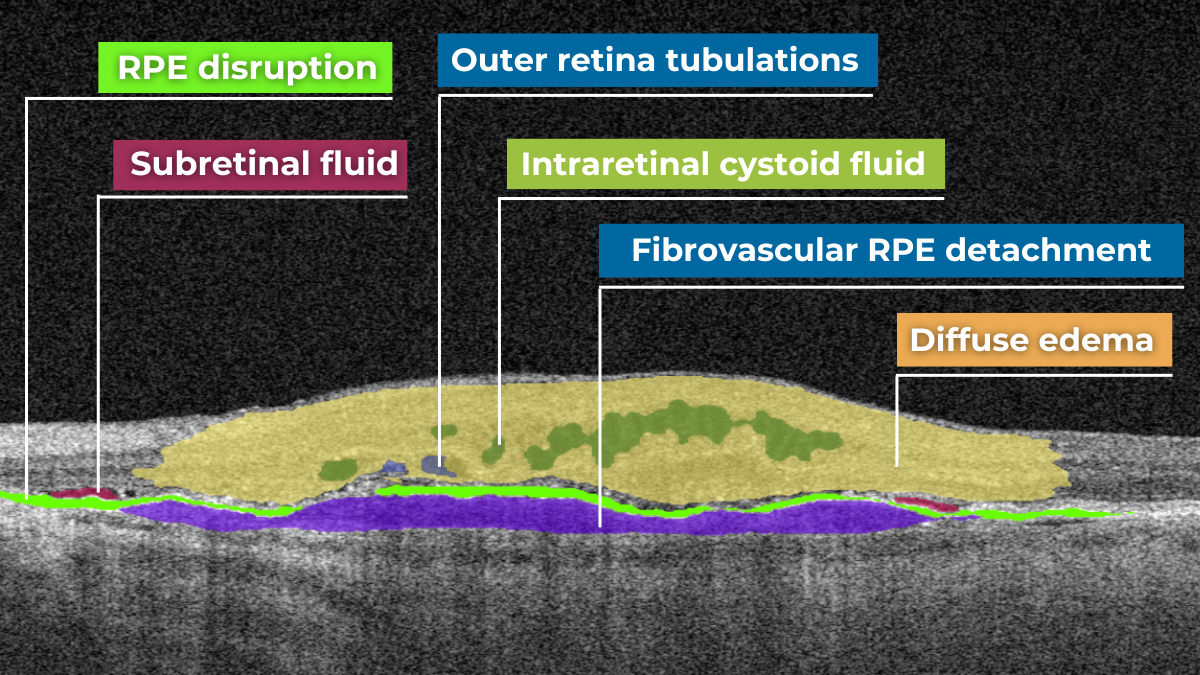
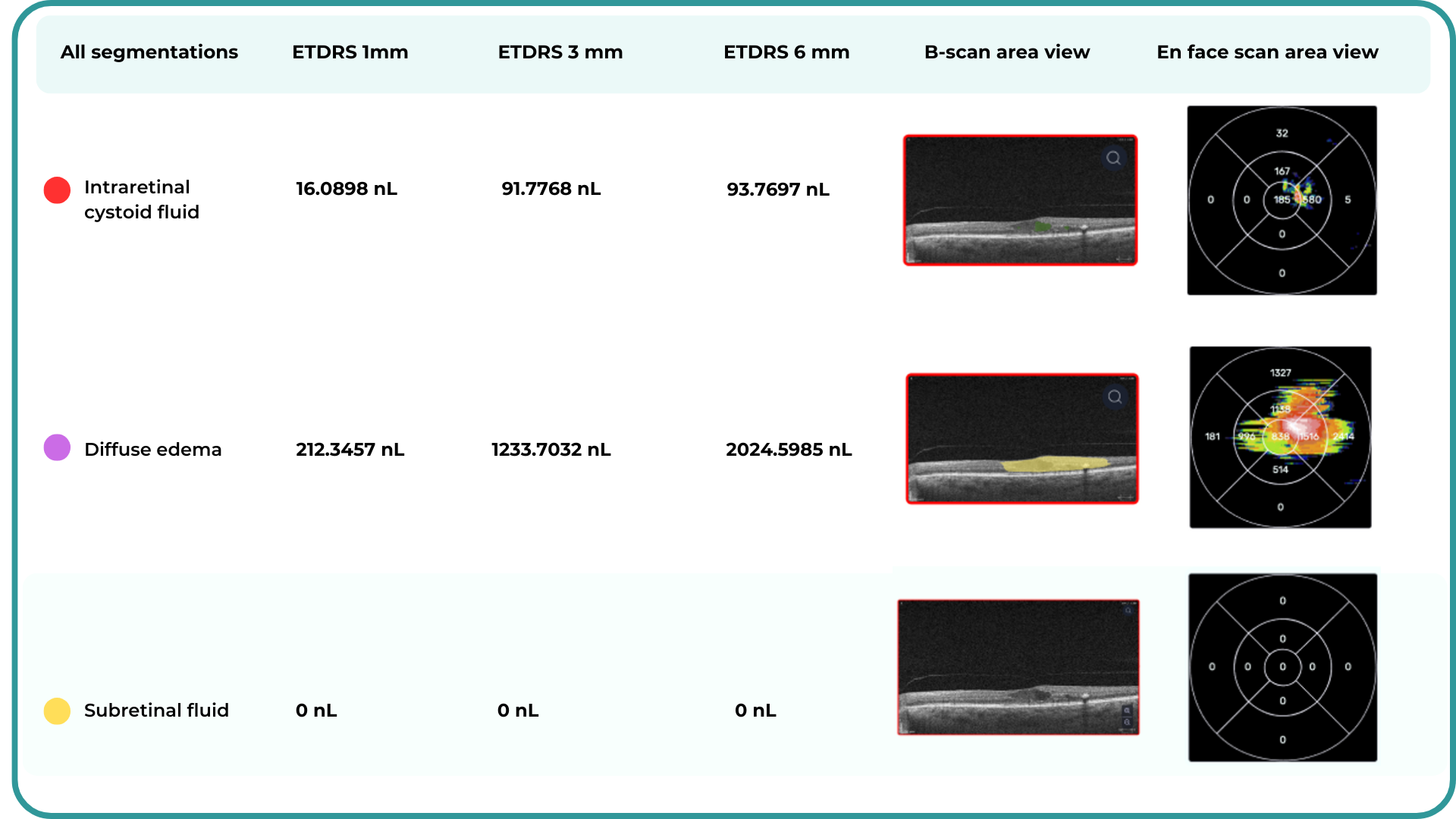
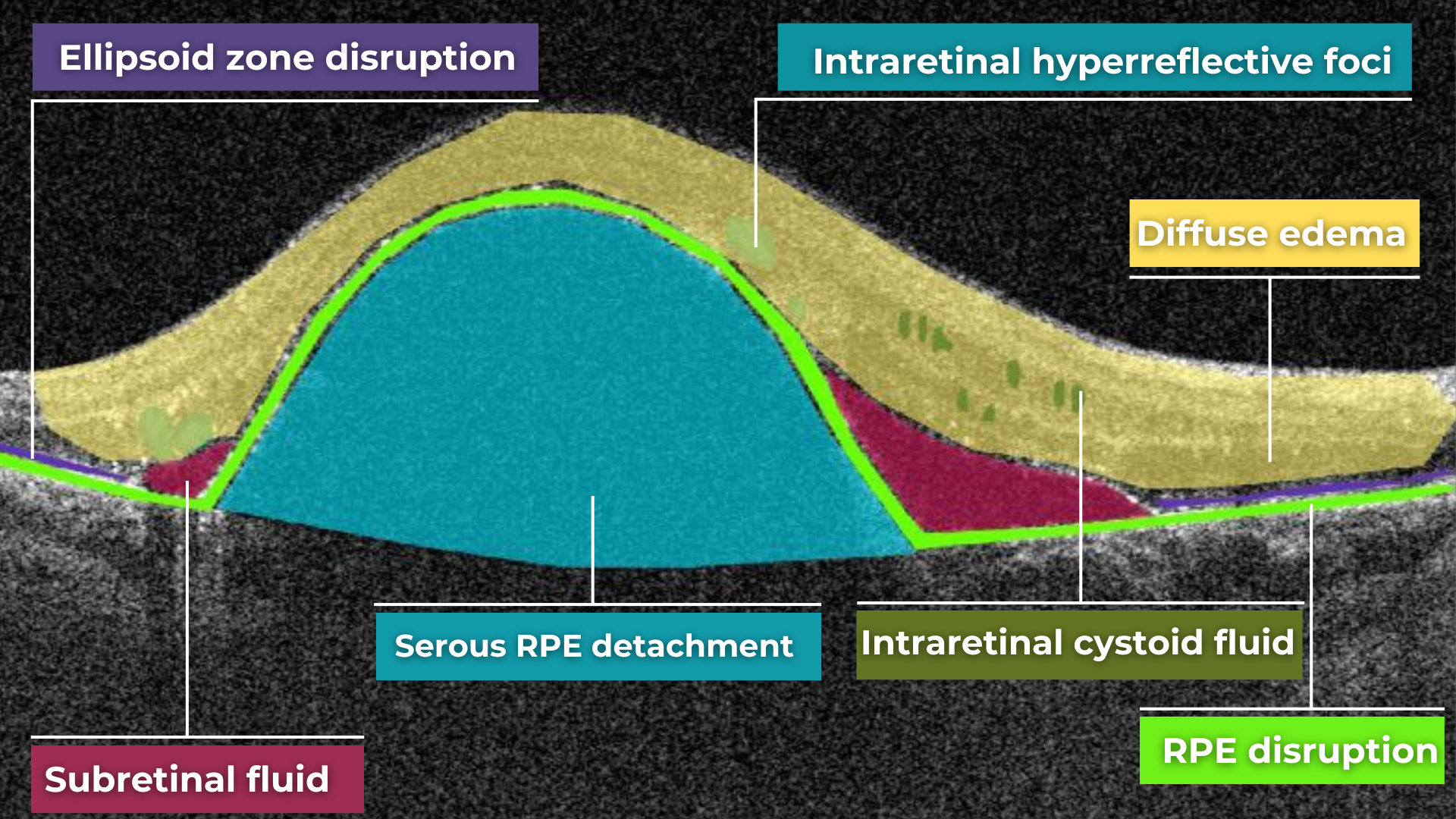
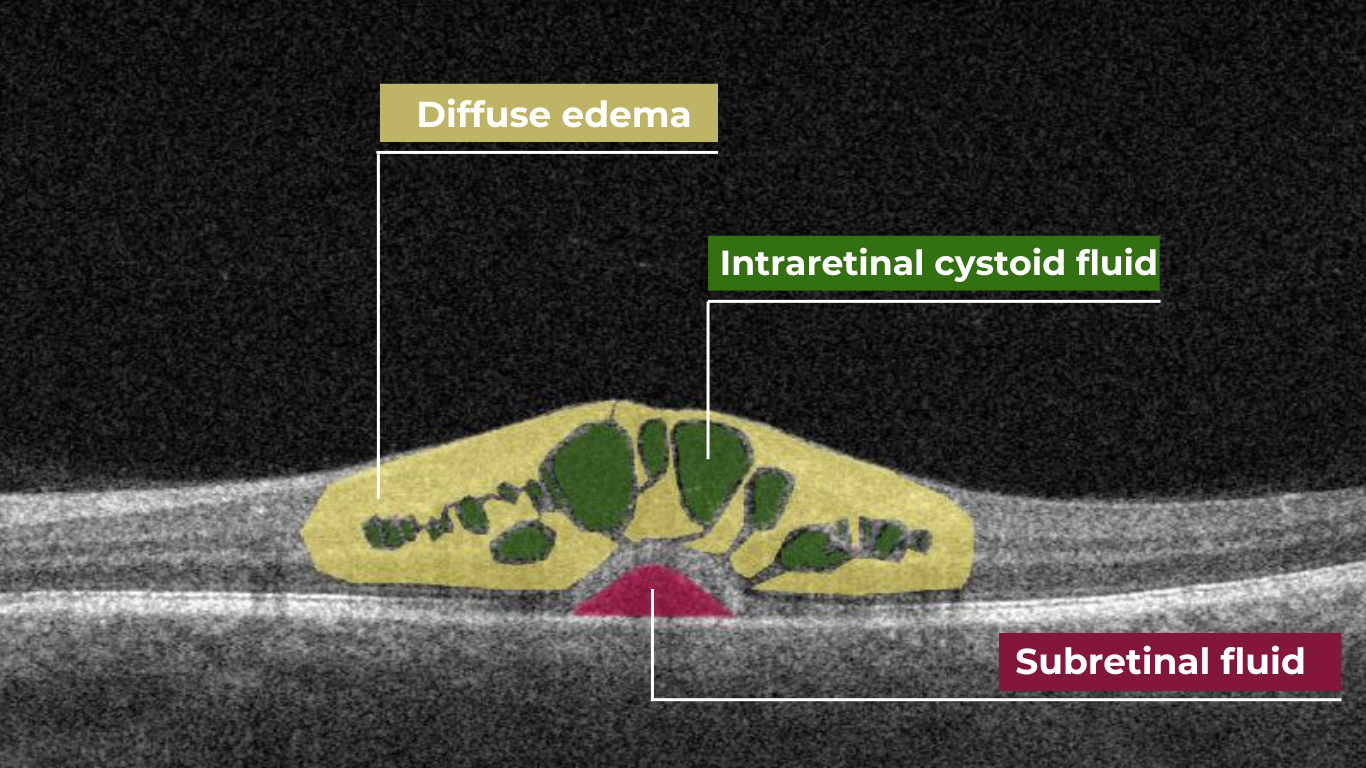
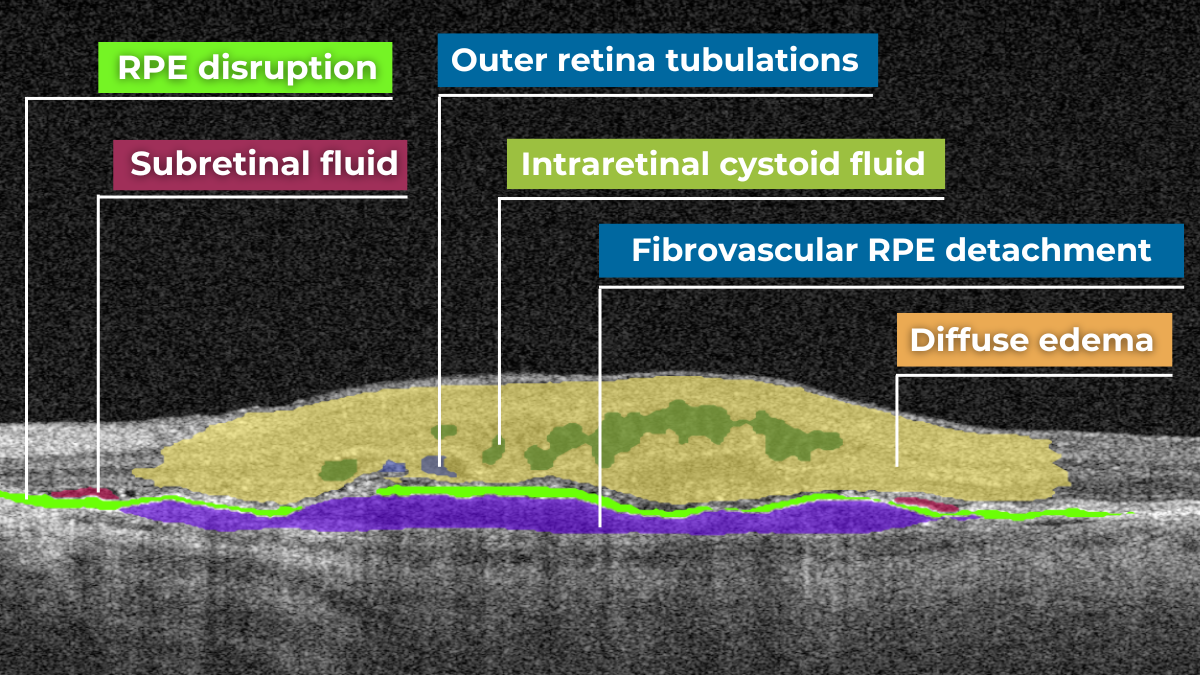
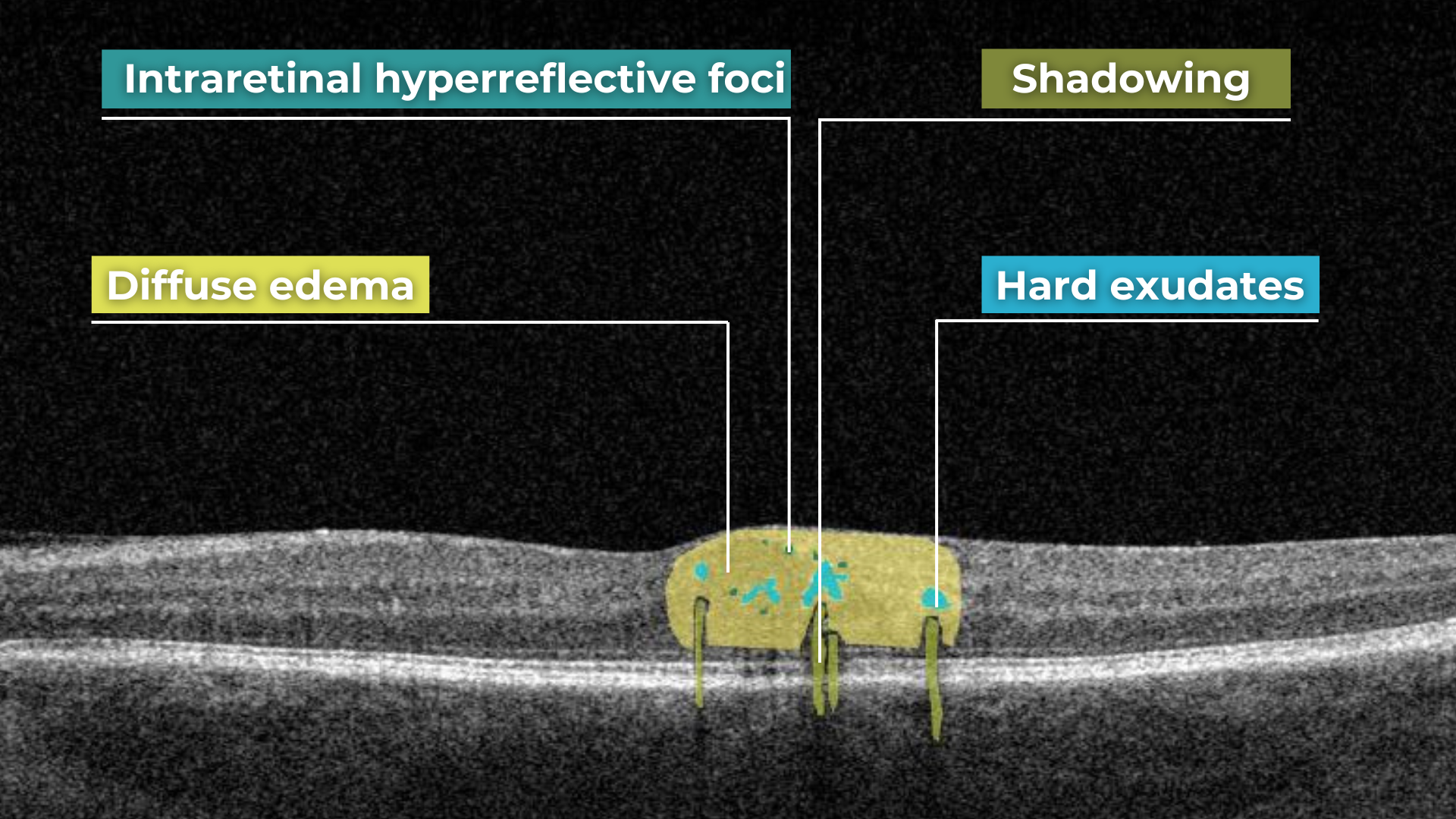
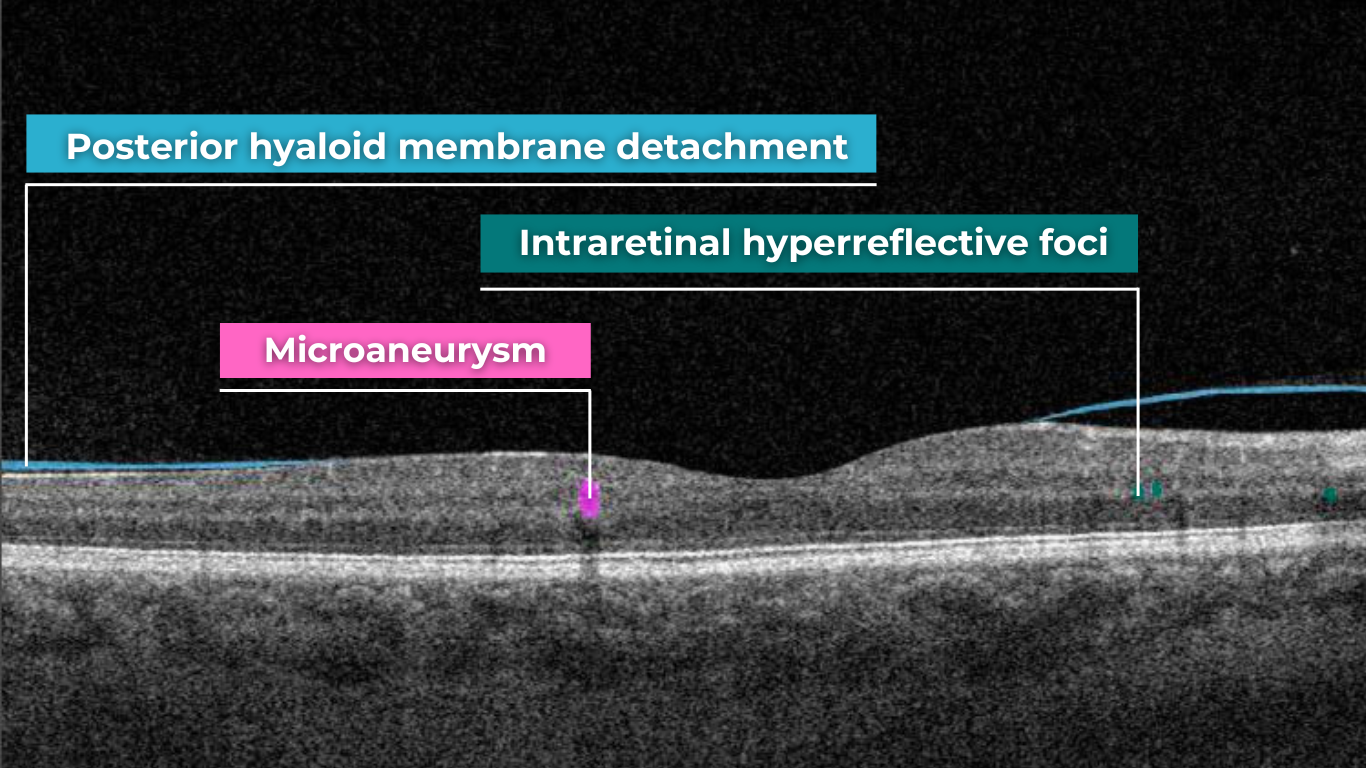
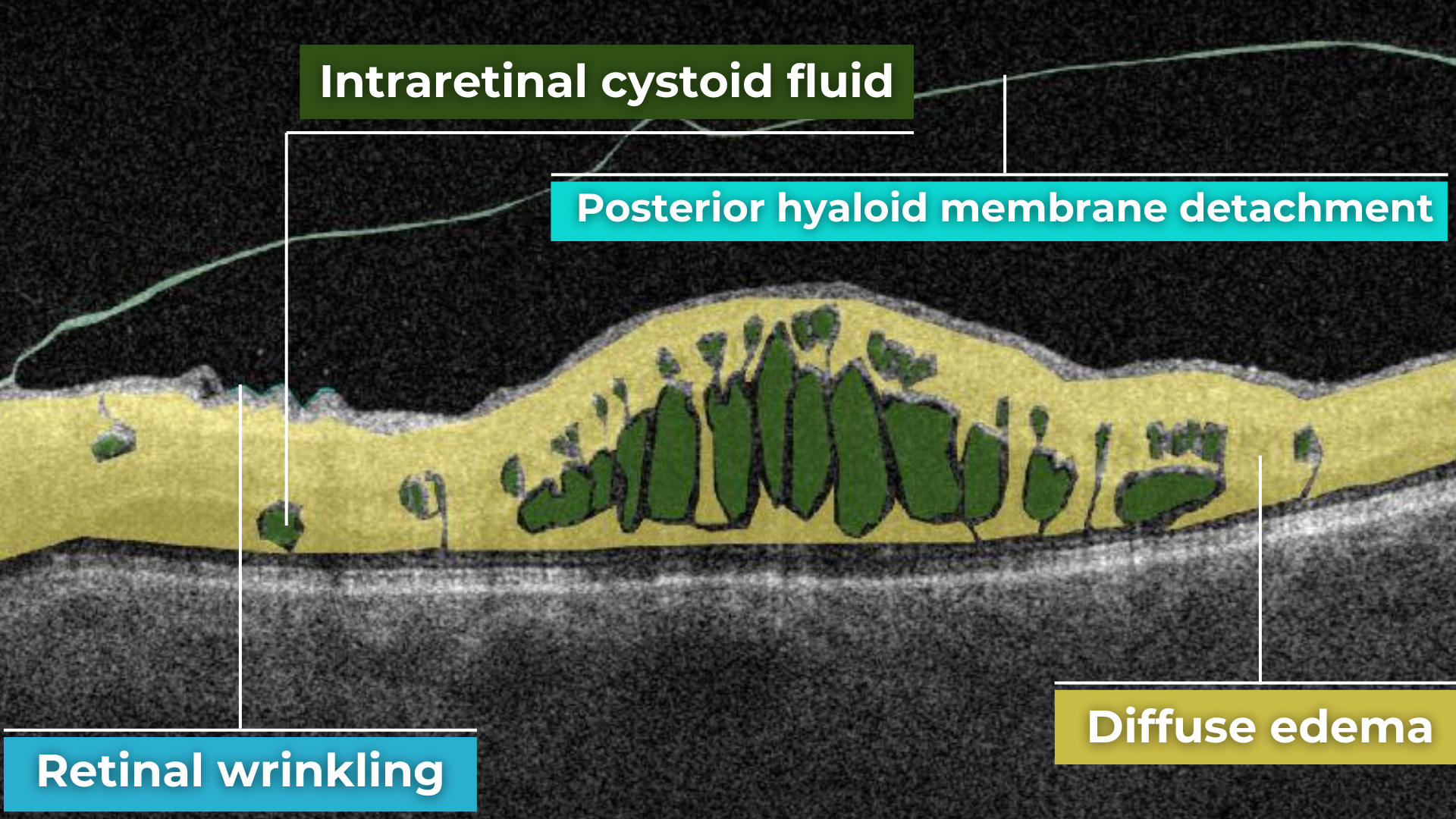
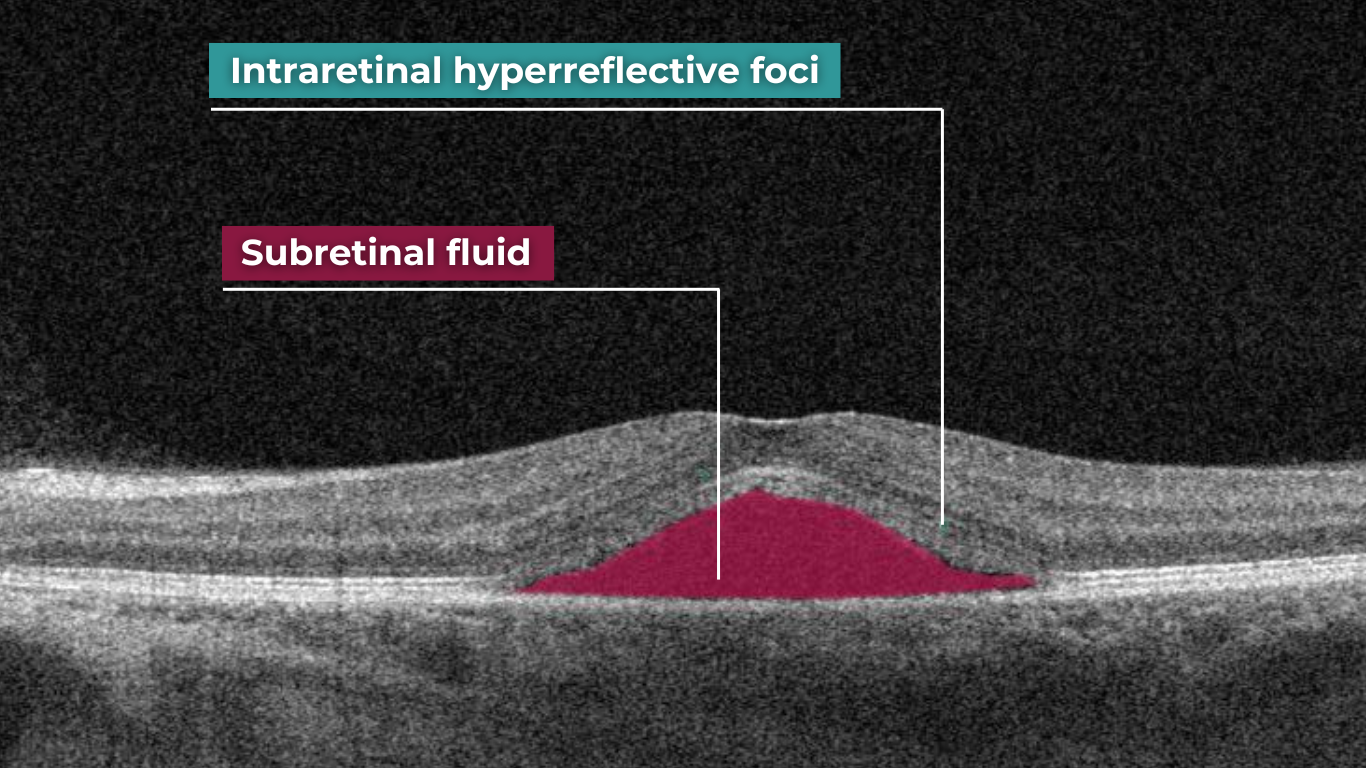
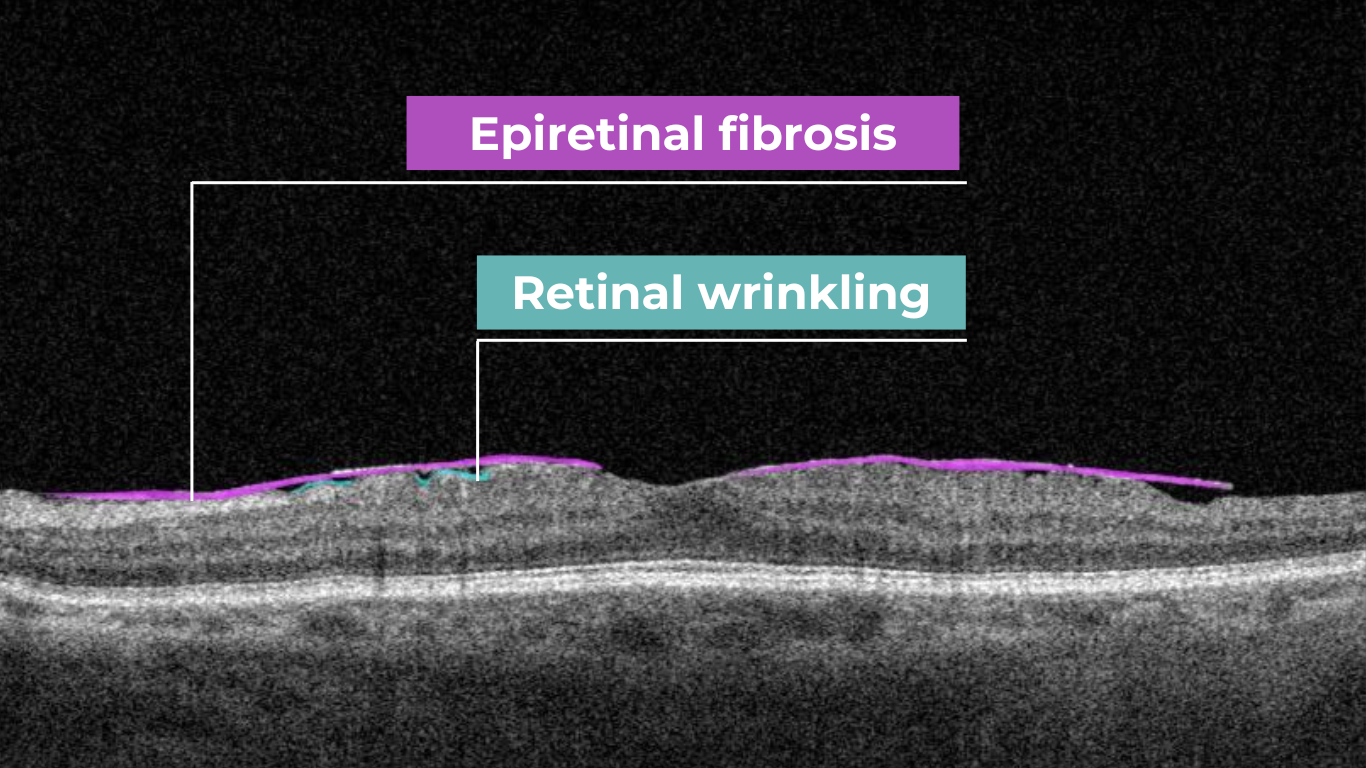
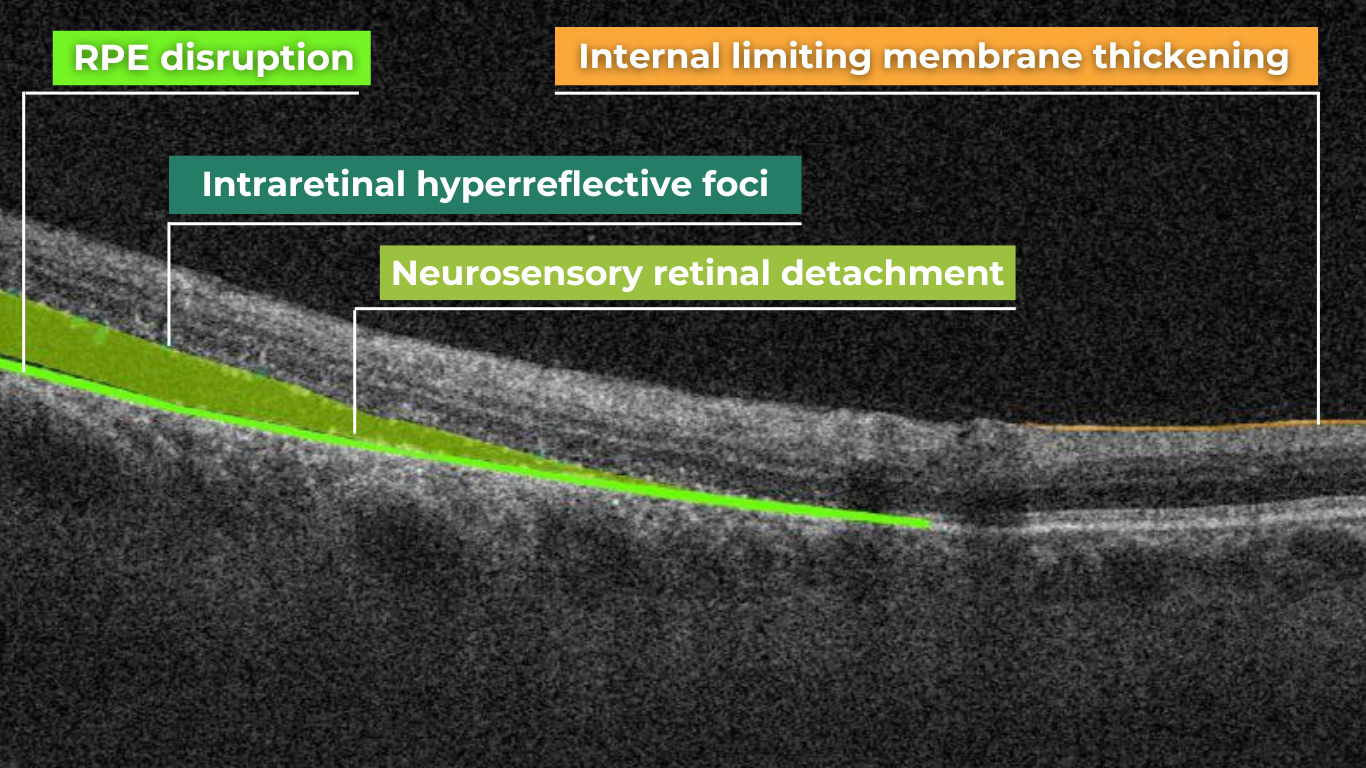
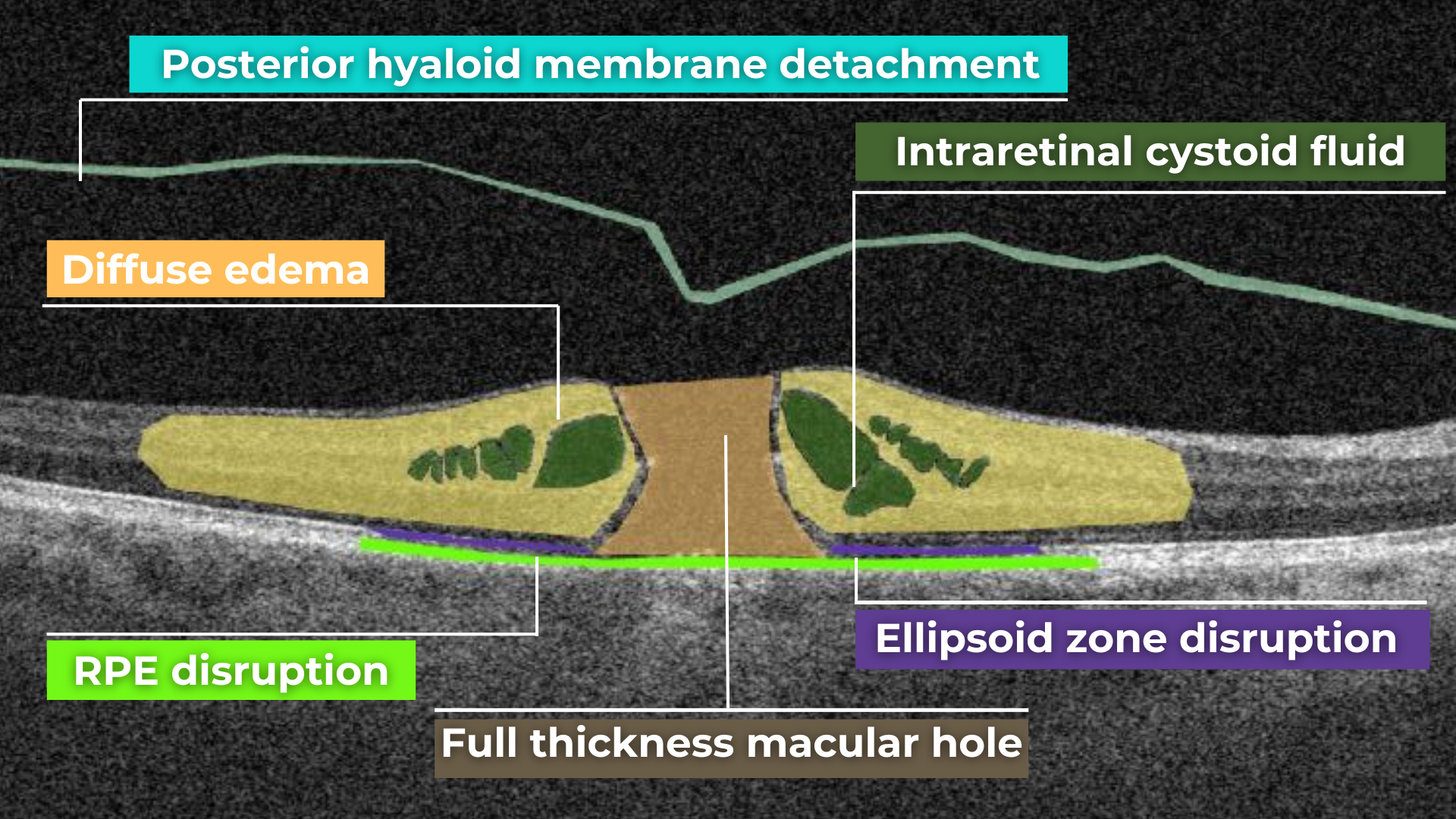
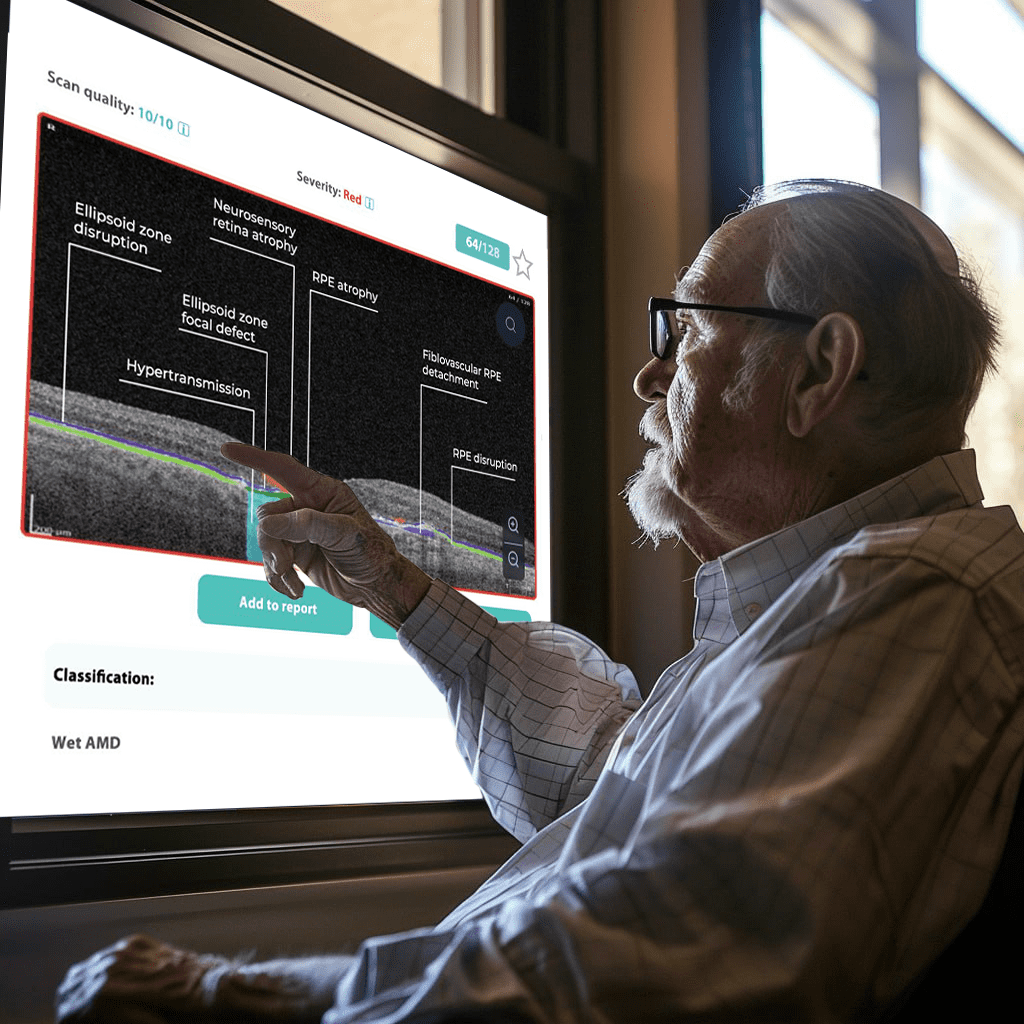
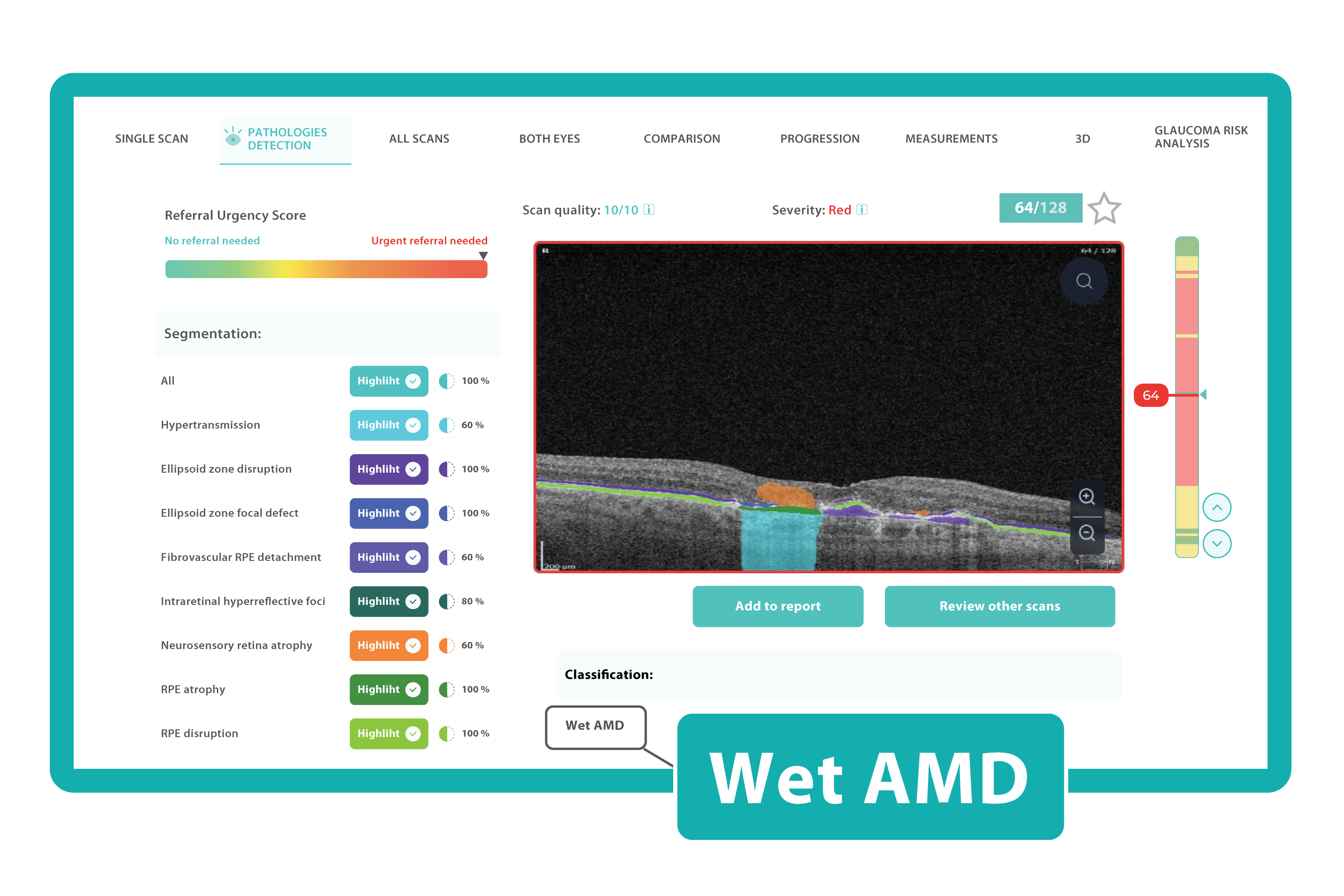
How does it work? Altris AI serves as a copilot, analyzing OCT scans in parallel to the eye care specialist. For instance, on this OCT scan, Altris AI detects Diffuse Edema, Floaters, Intraretinal Hyperreflective Foci, Posterior Hyaloid Membrane Detachment, RPE disruption, Shadowing, Hard Exudates, Intraretinal Cystoid Fluid.
- The classification in this case would be Diabetic Retinopathy.

With Altris AI, the process becomes significantly faster and more efficient. The AI algorithms can quickly analyze intricate details within the scans, providing clinicians with accurate and timely insights into the patient’s eye health.
Moreover, the use of Altris AI contributes to increased diagnostic accuracy. The algorithms are trained on vast datasets, learning to recognize subtle patterns and anomalies that may escape the human eye.
Thus, Altris AI recognizes 70+ retina pathologies and biomarkers, including DME, DR, GA, AMD, etc.

FDA-cleared AI for OCT Analysis
Try it yourself in our Demo Account or get a Brochure
Technologies in Optometry are paving the way to a new future where eye care specialists and AI will work together for better patient outcomes. AI will never be able to substitute eye care specialists because the final diagnosis must include clinical history, results of lab tests, and other diagnostic methods.
-

OCT Layers of Retina
 Maria Martynova
5 min.5 min.
Maria Martynova
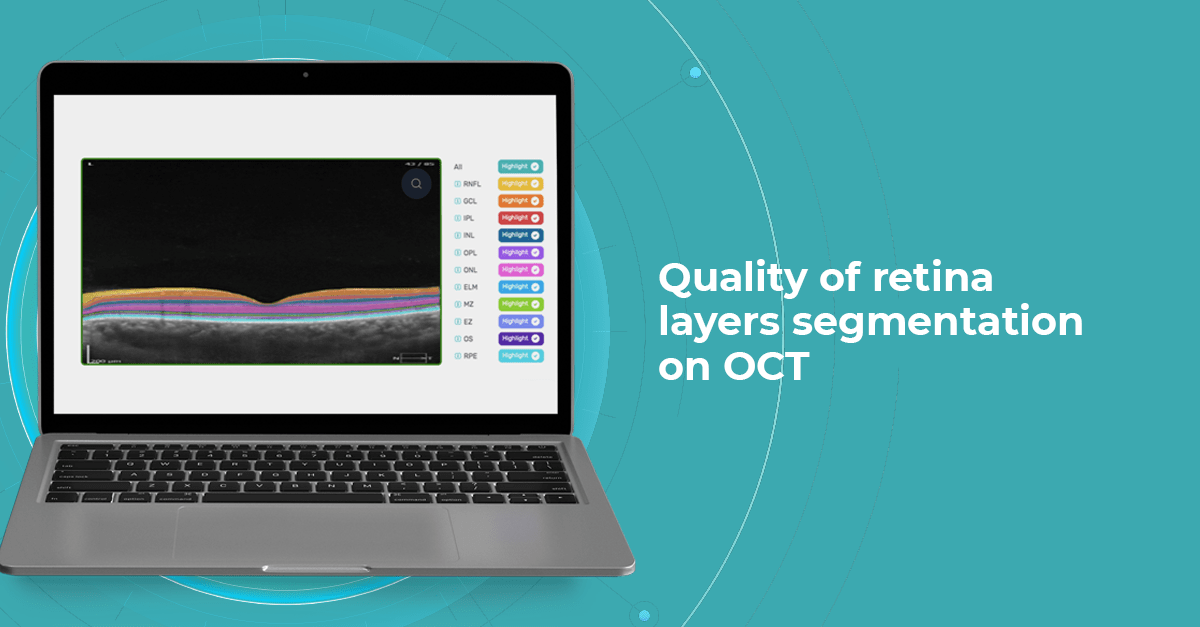
5 min.5 min.OCT Layers of Retina: modern approach to segmentation
The knowledge about macular retinal layer thicknesses and volume is an important diagnostic tool for any eye care professional today. The information about the macular retinal layers often correlates with the evaluation of severity in many pathologies.
Manual segmentation is extremely time-consuming and prone to numerous errors, which is why OCT equipment manufacturers use automatic macular retinal layer thickness segmentation.

Segment retina layers with AI
Yet, retina layer segmentation in different OCT equipment manufacturers as well as in different OCT models varies significantly. It is sometimes difficult even for an experienced ECP to find the correlations and track the pathology dynamics. The normative bases refer only to the thickness of the entire retina, they are not related to segmentation. However, if the segmentation is performed incorrectly by the machine, it will lead to an incorrect calculation of the thickness of the retina or its layers, and then the assessment will be incorrect.
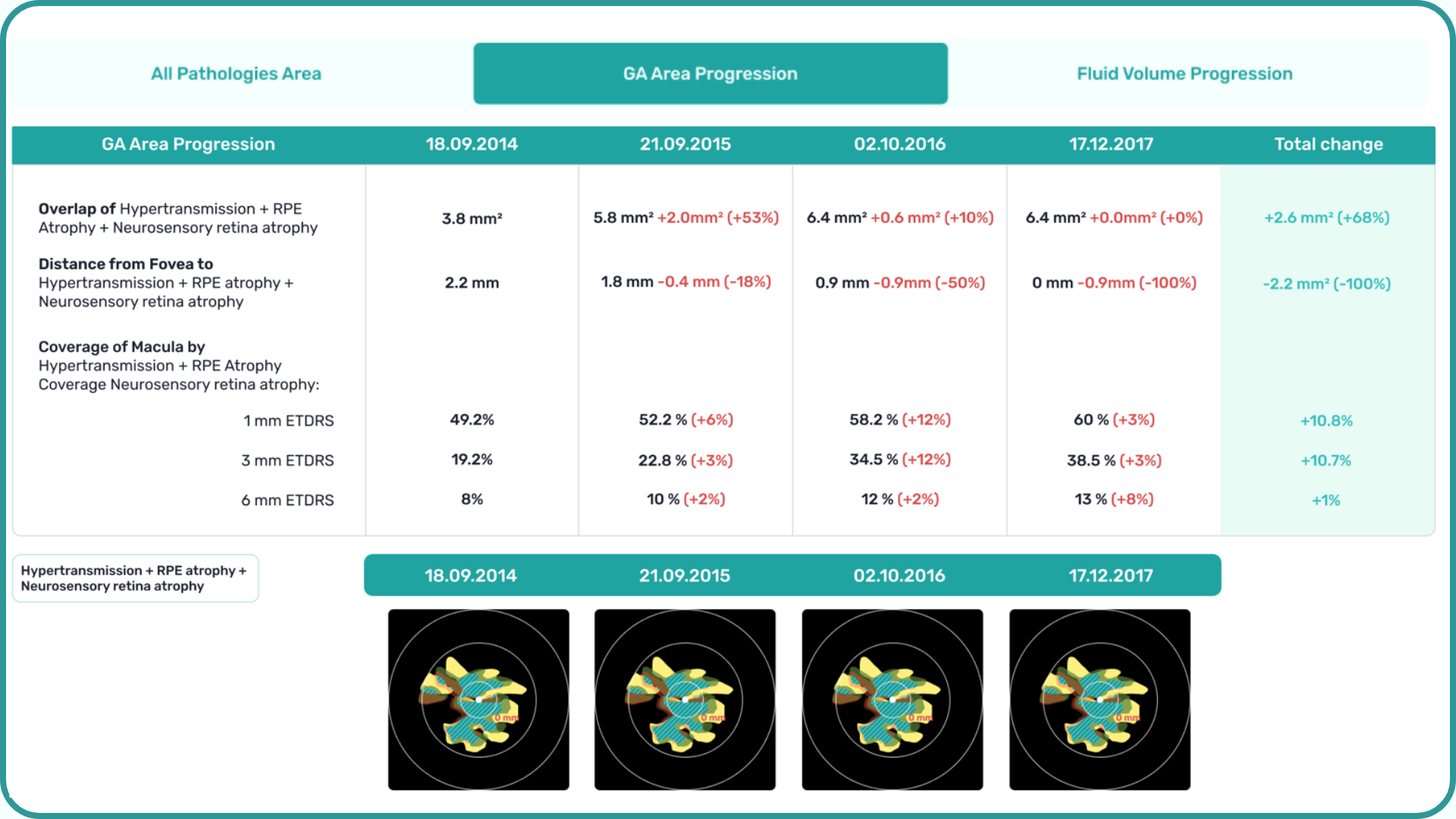
At Altris AI we aim to visualize retina layers for a more accurate understanding of pathological process localization. Such retina layers segmentation allows for defining the localization of the pathological process and tracing in dynamics the spread of the pathological process or the aftermath in the retina structure after its completion.
For instance, the EZ layer is important for vision loss forecasting.
OCT Manufacturers & Retina Layers Analysis
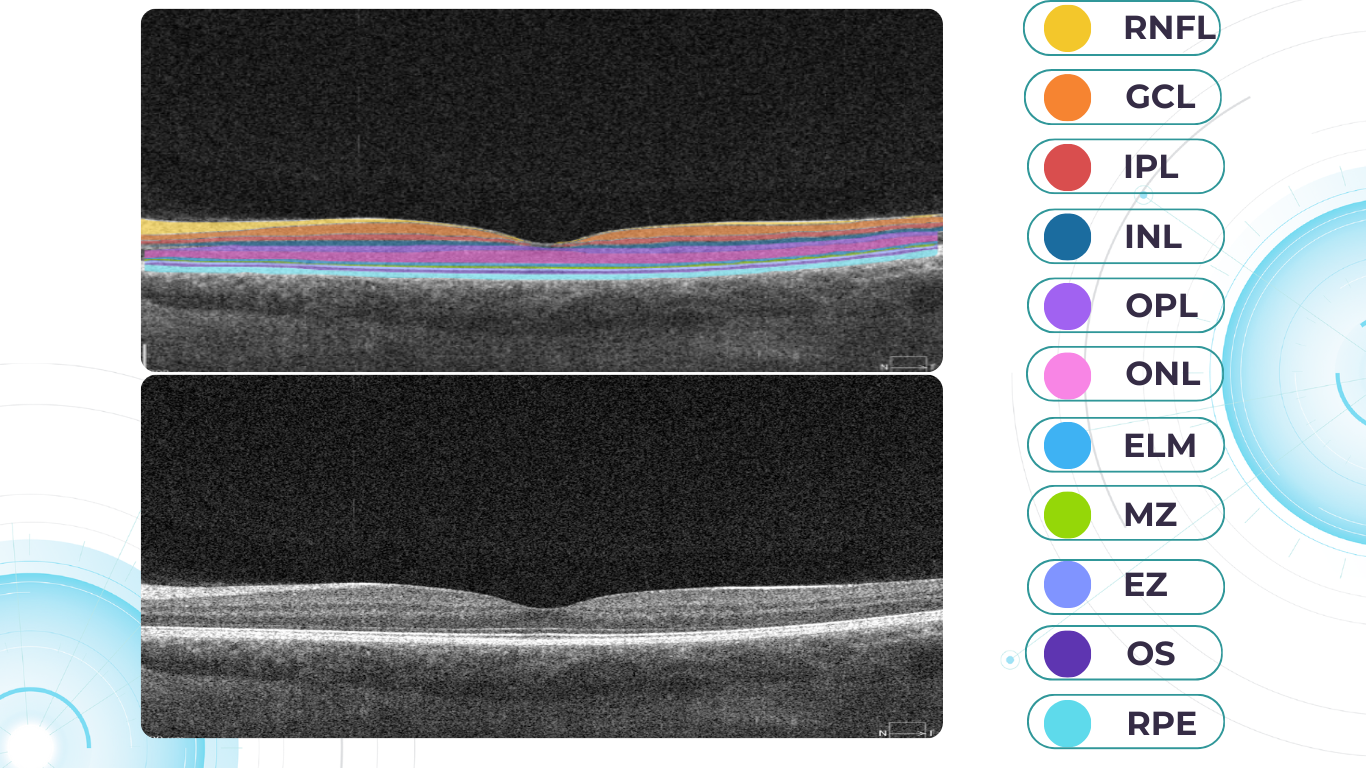
From 2010 most eye care specialists have used the same OCT International Nomenclature for Optical Coherence Tomography. OCT equipment manufacturers rely on this nomenclature for retina layer thickness calculation and most ophthalmologists use it as well. Here is a variant of OCT layer segmentation:

Taking into account retina structure, some layers can be united into complexes. For instance, the ganglion complex includes RNFL, ganglion cell layer & OPL.
Let’s take a look at various OCT equipment manufacturers and the way they perform retina layer segmentation analysis.
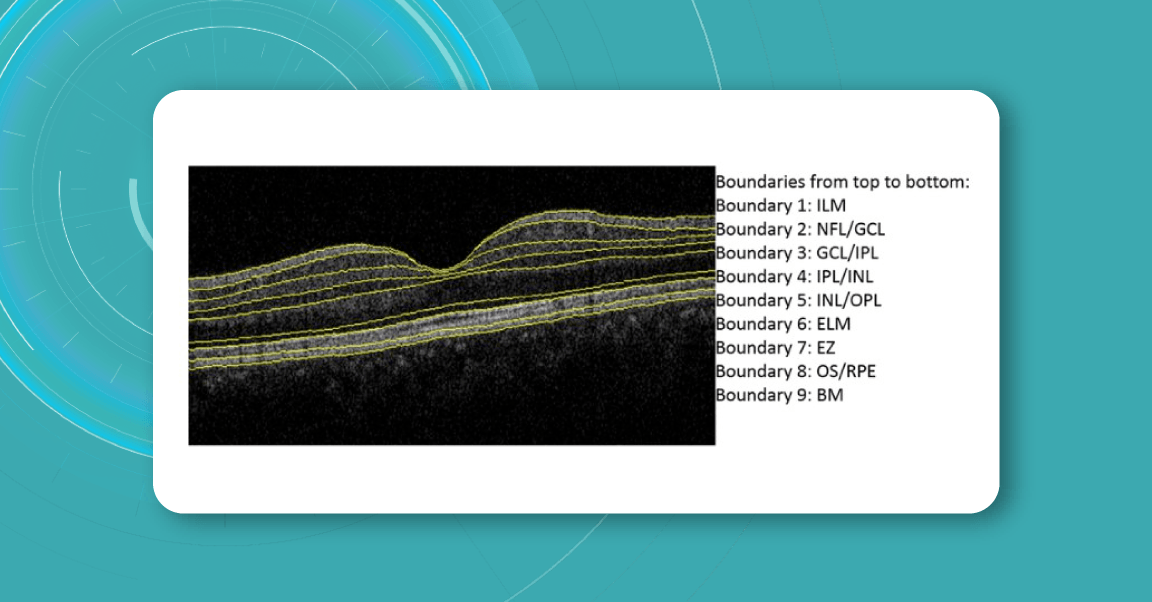
For instance, here is how Topcon Advanced Boundary Segmentation (TABSTM) automated segmentation differentiates between nine intraretinal boundaries:
- ILM
- NFL/GCL,
- GCL/IPL,
- IPL/INL,
- INL/OPL,
- ELM
- EZ
- OS/RPE
- BM

Zeiss CIRRUS uses two approaches to retina layer segmentation.

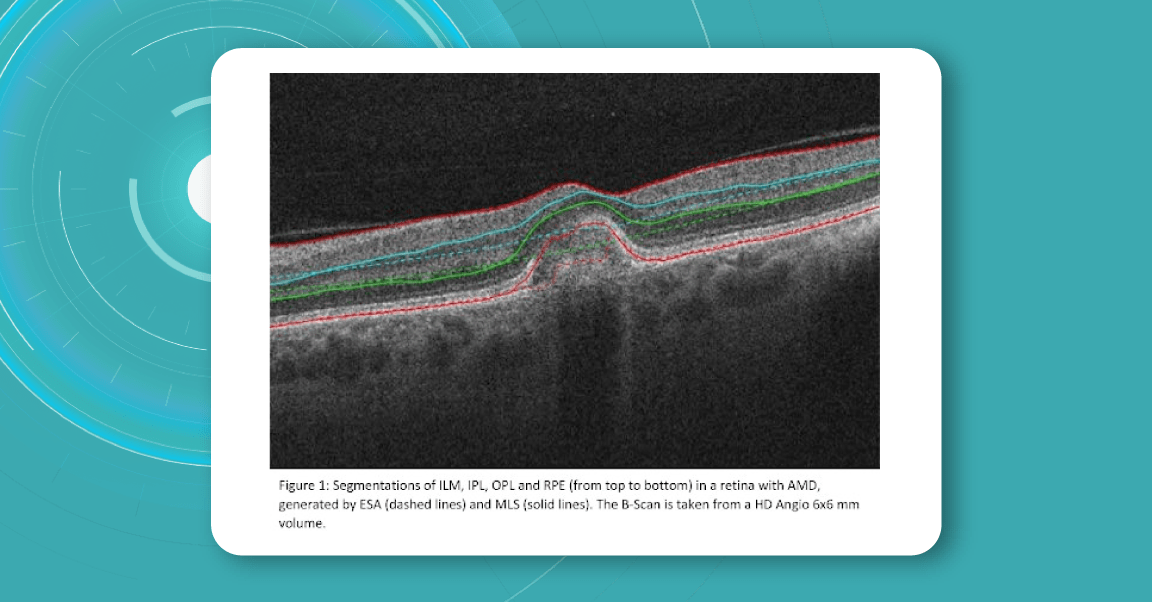
The existing segmentation algorithm (ESA) in CIRRUS estimates the positions of the inner plexiform layer (IPL) and outer plexiform layer (OPL) based on the internal limiting membrane (ILM) and retinal pigment epithelium (RPE). To improve the accuracy of the segmentation of these layers, a multi-layer segmentation algorithm (MLS) was introduced, it truly segments layers instead of estimating their position.
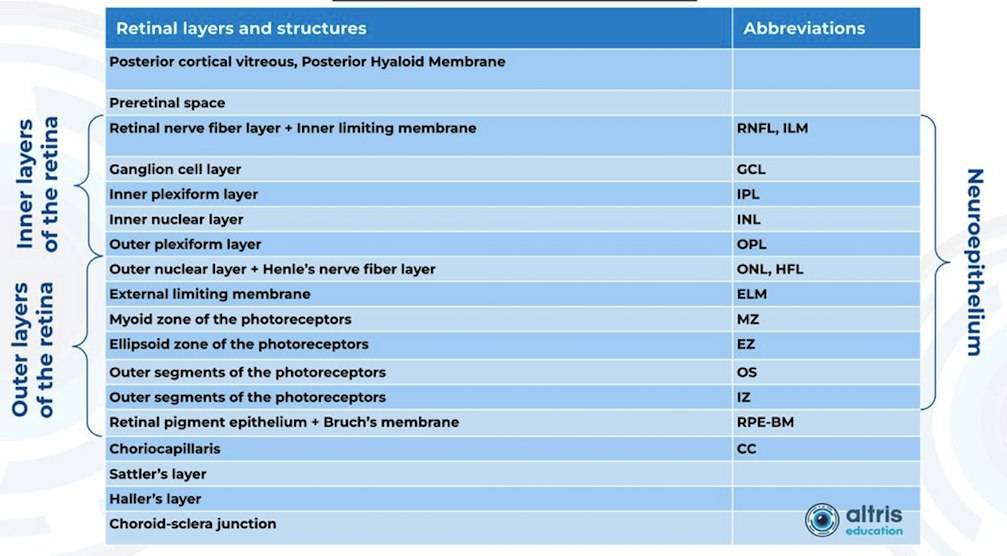
Heidelberg Engineering offers to learn about the following inner and outer retina layers on their website. There are 10 retina layers according to Heidelberg, and they are the following:
- ILM
- RNFL
- GCL
- IPL
- INL
- OPL
- ONL
- ELM
- PR
- RPE
- BM
- CC
- CS
Retina Layers on OCT with Altris AI: More Clinical Insights
Altris AI segments 12 retina layers and measures their thickness with maximum precision. Here are the OCT retina layers we work with:
-
RNFL – Retinal Nerve Fiber Layer
Contains ganglion cell axons; thinning is a key marker for glaucoma.
Measuring its thickness helps detect and monitor glaucomatous damage. -
GCL – Ganglion Cell Layer
Composed of ganglion cell bodies; damage here indicates neurodegeneration.
Thickness assessment aids in the early diagnosis of glaucoma and optic neuropathies. -
IPL – Inner Plexiform Layer
The site of synapses between bipolar and ganglion cells is vital for signal relay.
Changes in thickness can reflect inner retinal dysfunction, especially in diabetic retinopathy. -
INL – Inner Nuclear Layer
Houses bipolar, amacrine, and horizontal cell bodies are essential for visual processing.
Swelling or thinning may indicate retinal vascular disease or macular edema. -
OPL – Outer Plexiform Layer
Where photoreceptors connect to bipolar cells; disruptions may signal early maculopathy.
Thickness alterations can be associated with retinal ischemia or structural disorganization. -
ONL – Outer Nuclear Layer
Contains the nuclei of photoreceptors; thinning may indicate photoreceptor loss.
Tracking its thickness supports evaluation of photoreceptor integrity in degenerative diseases. -
ELM – External Limiting Membrane
A structural boundary supporting photoreceptor alignment and health.
Integrity and thickness are indicators of photoreceptor viability in macular disorders. -
MZ – Myoid Zone of Photoreceptors
Contains organelles like the endoplasmic reticulum; changes may reflect early photoreceptor stress.
Subtle thickness variations may serve as early markers of photoreceptor damage. -
EZ – Ellipsoid Zone of Photoreceptors
A mitochondrial-rich layer critical for photoreceptor energy supply; disruption suggests dysfunction.
Its thickness and continuity are key indicators of visual potential and retinal health. -
OS – Outer Segment
Responsible for light detection; damage here impairs visual transduction.
Measuring OS thickness is essential for assessing photoreceptor function and recovery. -
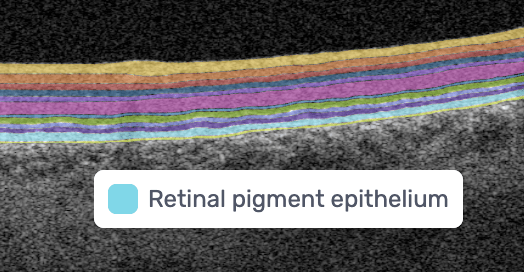
RPE – Retinal Pigment Epithelium
Supports photoreceptors and waste removal; essential in maintaining retinal health.
Changes in RPE thickness can indicate AMD, central serous chorioretinopathy, and other retinal diseases. -
BM – Bruch’s Membrane
A barrier beneath the RPE; thickening or breaks are early signs of AMD.
Assessing thickness helps detect early signs of age-related macular degeneration and choroidal changes.
Why is accurate retina layer segmentation important?
Retina layers segmentation helps eye care professionals to understand which pathology to consider in the first turn. For instance, changes in RPE and PR signify the development of Macular Degeneration.
Often such changes can also inform eye care specialists about the development of pathologies that lead to blindness, such as glaucoma, AMD, and Diabetic Retinopathy.
- Early Glaucoma Detection
Historically, evaluation of early glaucomatous change has focused mostly on optic disk changes. Modalities such as optical coherence tomography (OCT), confocal scanning laser ophthalmoscopy (HRT) or scanning laser polarimetry (GDx) with specially developed software algorithms have been used to quantitatively assess such changes. However, glaucomatous damage is primarily focused on retinal ganglion cells, which are particularly abundant in the peri-macular region (the only retinal area with a ganglion cell layer more than 1 layer thick), constituting, together with the nerve fiber layer, up to 35% of retinal macular thickness.
Therefore, glaucomatous changes causing ganglion cell death could potentially result in a reduction of retinal macular thickness. Indeed, by employing specially developed algorithms to analyze OCT scans, previous studies have reported that glaucoma, even during the early stage, results in the thinning of inner retinal layers at the macular region.
According to this study, the RNFL, GCL, and IPL levels out of all the retinal layers, the inner-most layers of the retina: the retinal nerve fiber layer (RNFL), ganglion cell layer (GCL), and inner plexiform layer (IPL) show the best discriminative power for glaucoma detection. Among these, the RNFL around the circumpapillary region has shown great potential for discrimination. The automatic detection and segmentation of these layers can be approached with different classical digital image processing techniques.

Segment retina layers with AI
- Detection of AMD
This first population-based study on spectral-domain optical coherence tomography-derived retinal layer thicknesses in a total of ∼1,000 individuals provides insights into the reliability of auto-segmentation and layer-specific reference values for an older population.
The findings showed a difference in thicknesses between early AMD and no AMD for some retinal layers, suggesting these as potential imaging biomarkers. When comparing layer thicknesses between early AMD and no AMD (822 eyes, 449 participants), the retinal pigment epithelium/Bruch’s membrane complex demonstrated a statistically significant thickening, and photoreceptor layers showed a significant thinning.
- Detection of DR
The depth and spatially resolved retinal thickness and reflectance measurements are potential biomarkers for the assessment and monitoring of Diabetic Retinopathy, one of the key reasons for blindness around the globe.
For instance, this study confirmed that decreased RNFL thickness and increased INL/OPL thickness in diabetics without DR or with initial DR suggest early alterations in the inner retina. On the contrary, the outer retina seems not to be affected at the early stages of DM. Automatic intraretinal layering by SD-OCT may be a useful tool to diagnose and monitor early intraretinal changes in DR.
Conclusion:
OCT layer segmentation is crucial for the accurate detection of pathologies in the eye, especially in the field of ophthalmology and medical imaging. Here are several reasons why it is important:
Precise Diagnosis: Retina layer segmentation provides a detailed map of the different retinal layers, which helps in the precise diagnosis of various eye conditions. It allows clinicians to identify the exact location of abnormalities, such as cysts, hemorrhages, or lesions, within the retina.
Quantitative Analysis: It enables quantitative analysis of retinal structures. By measuring the thickness, volume, and other characteristics of specific layers, clinicians can assess the severity and progression of diseases like diabetic retinopathy, macular degeneration, and glaucoma.
Early Detection: Some retinal pathologies manifest in specific layers of the retina before becoming visible on a fundus photograph. Retina layer segmentation can help detect these changes at an early stage, potentially leading to earlier intervention and improved outcomes.
Treatment Planning: Knowing the precise location of pathologies within the retina’s layers can aid in the planning of treatment strategies. For example, in cases of macular holes or retinal detachment, surgeons can use this information to guide their procedures.
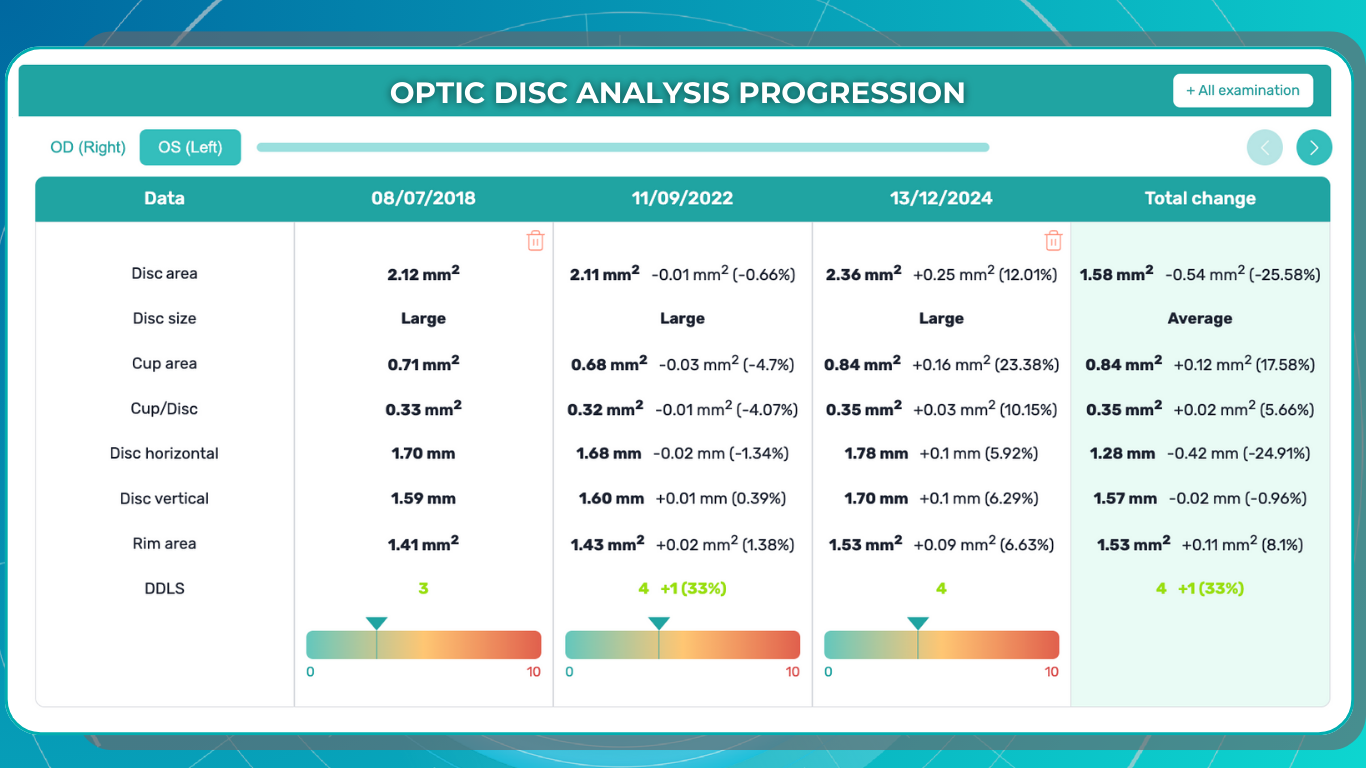
Monitoring Disease Progression: Retina layer segmentation is valuable for monitoring how retinal diseases progress over time. Changes in the thickness or integrity of specific layers can be tracked to assess the effectiveness of treatments or the worsening of conditions.

























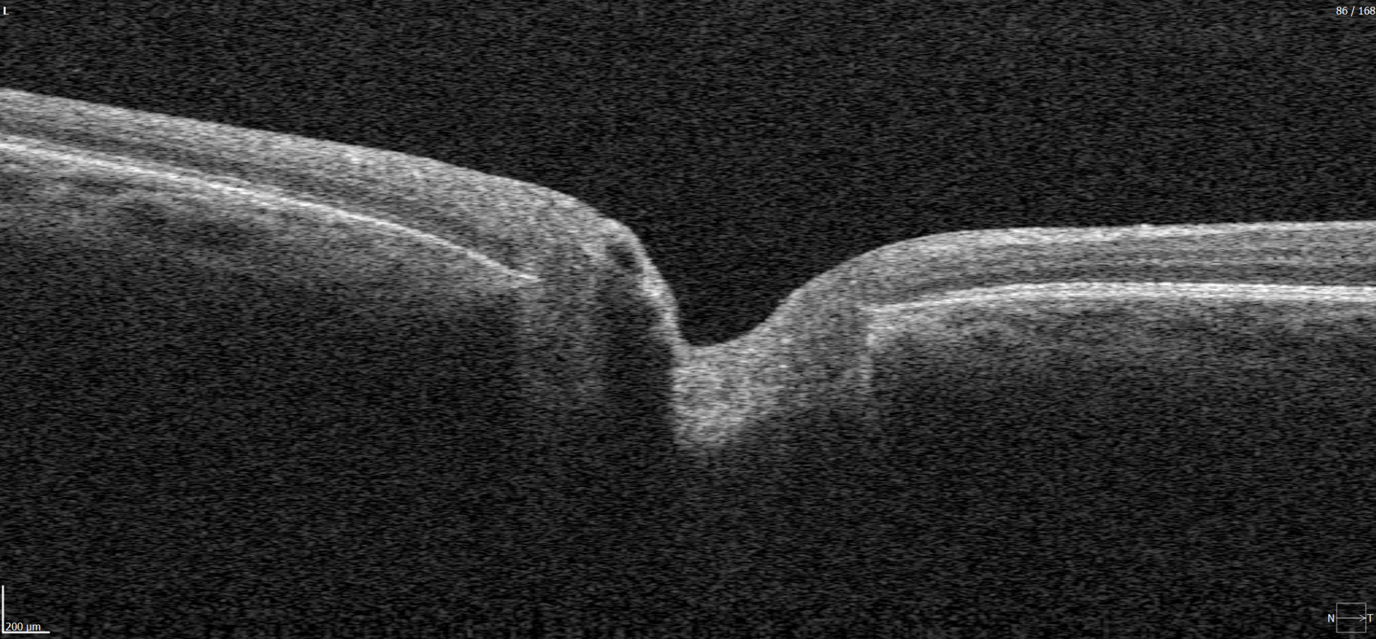
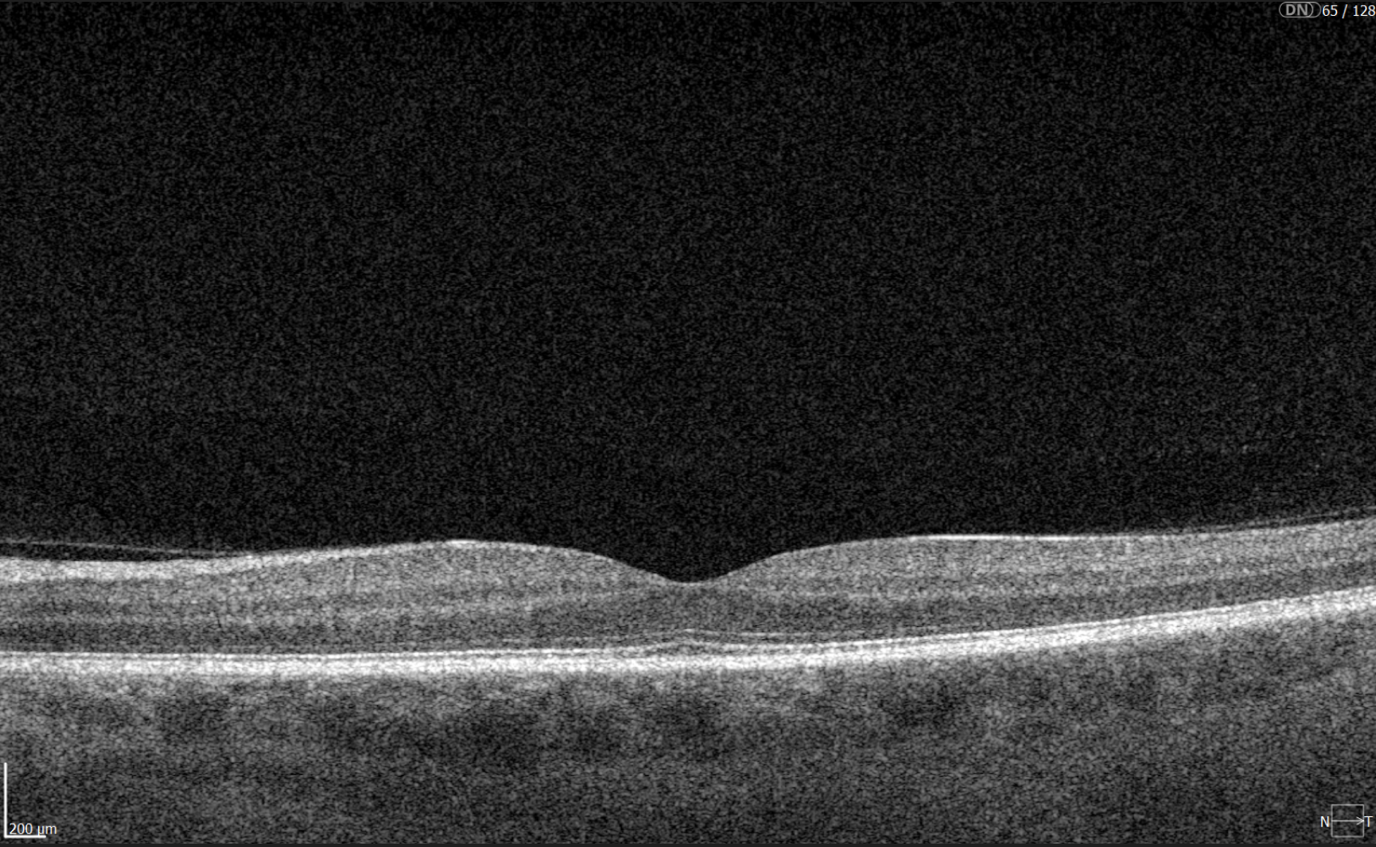
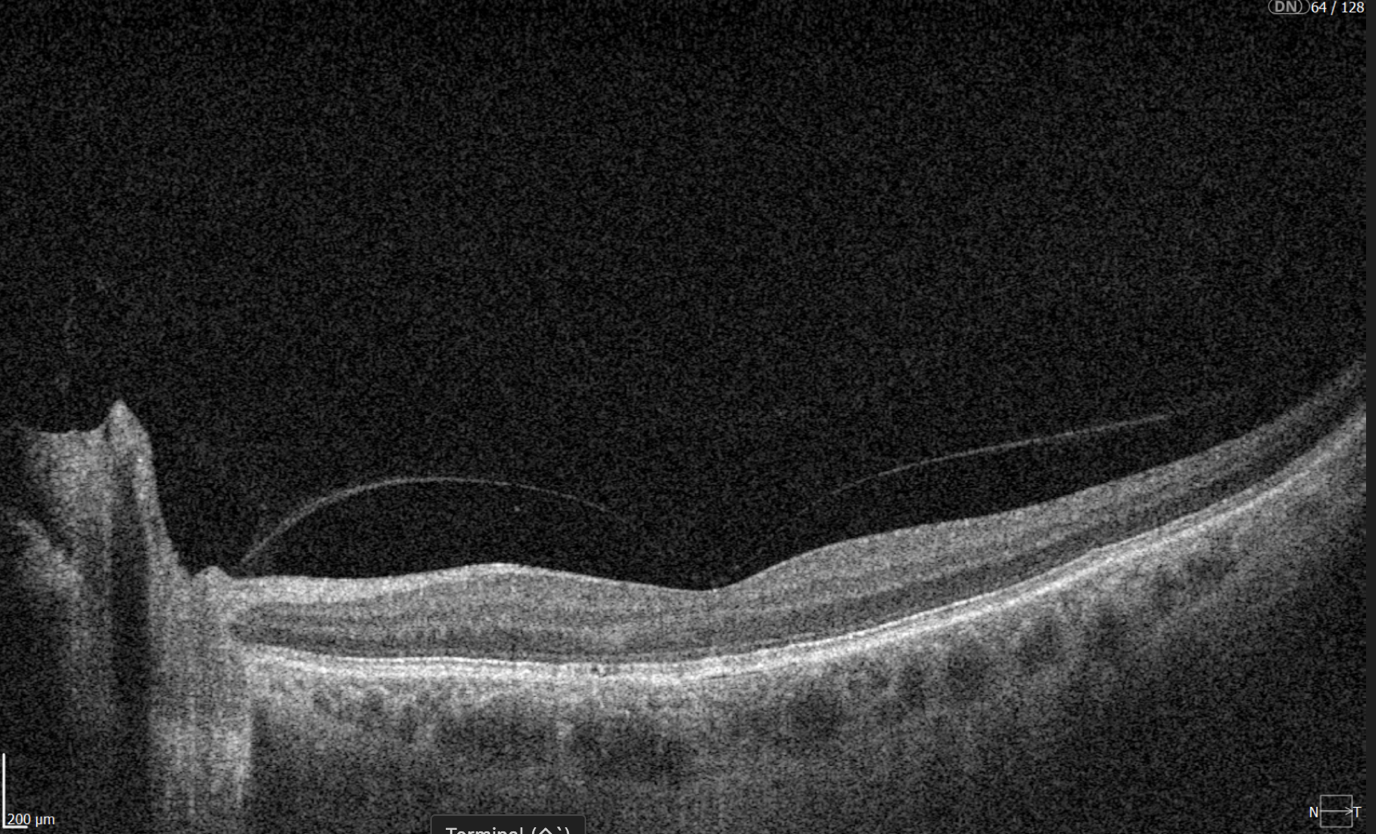
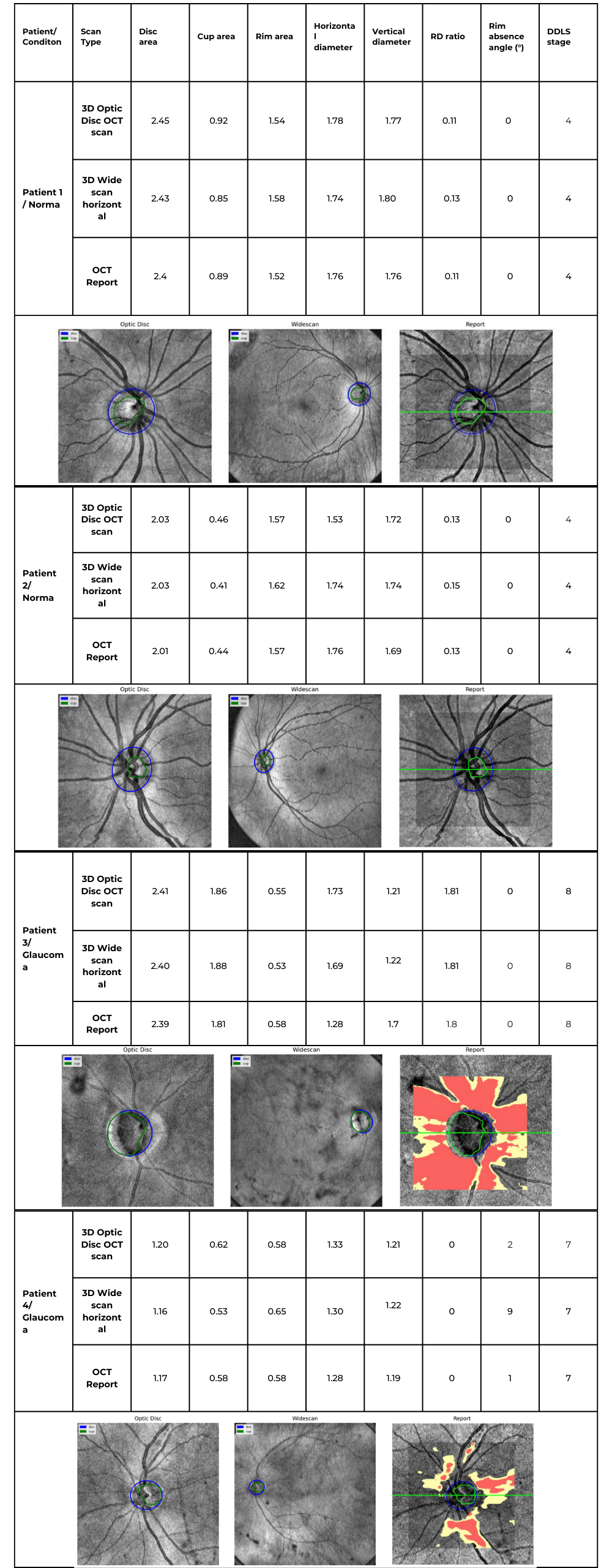
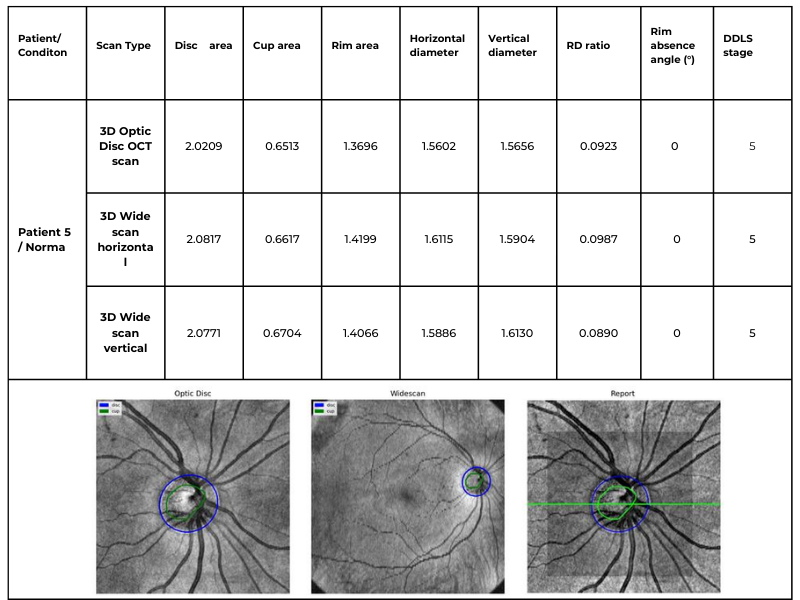
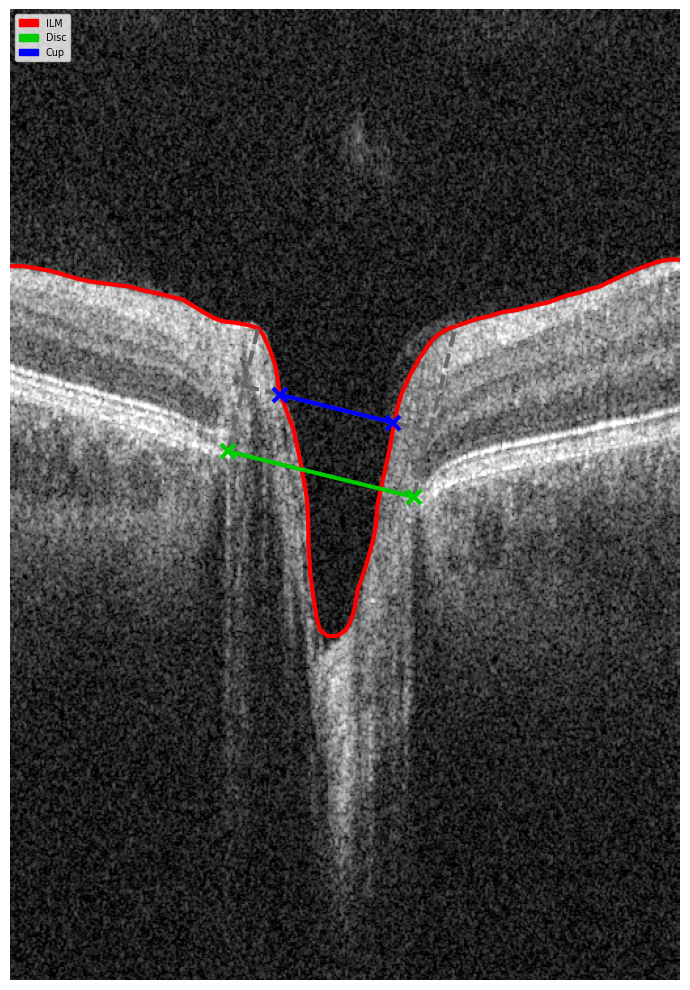
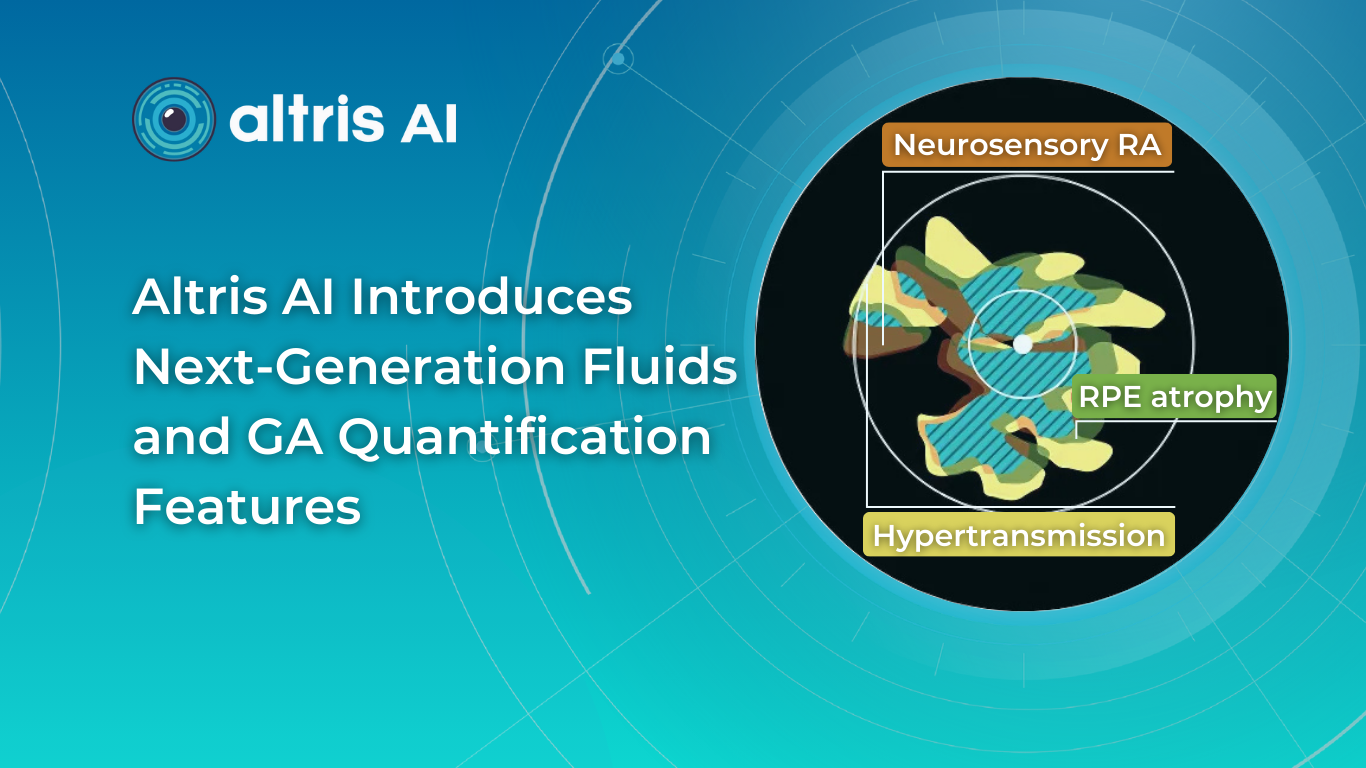
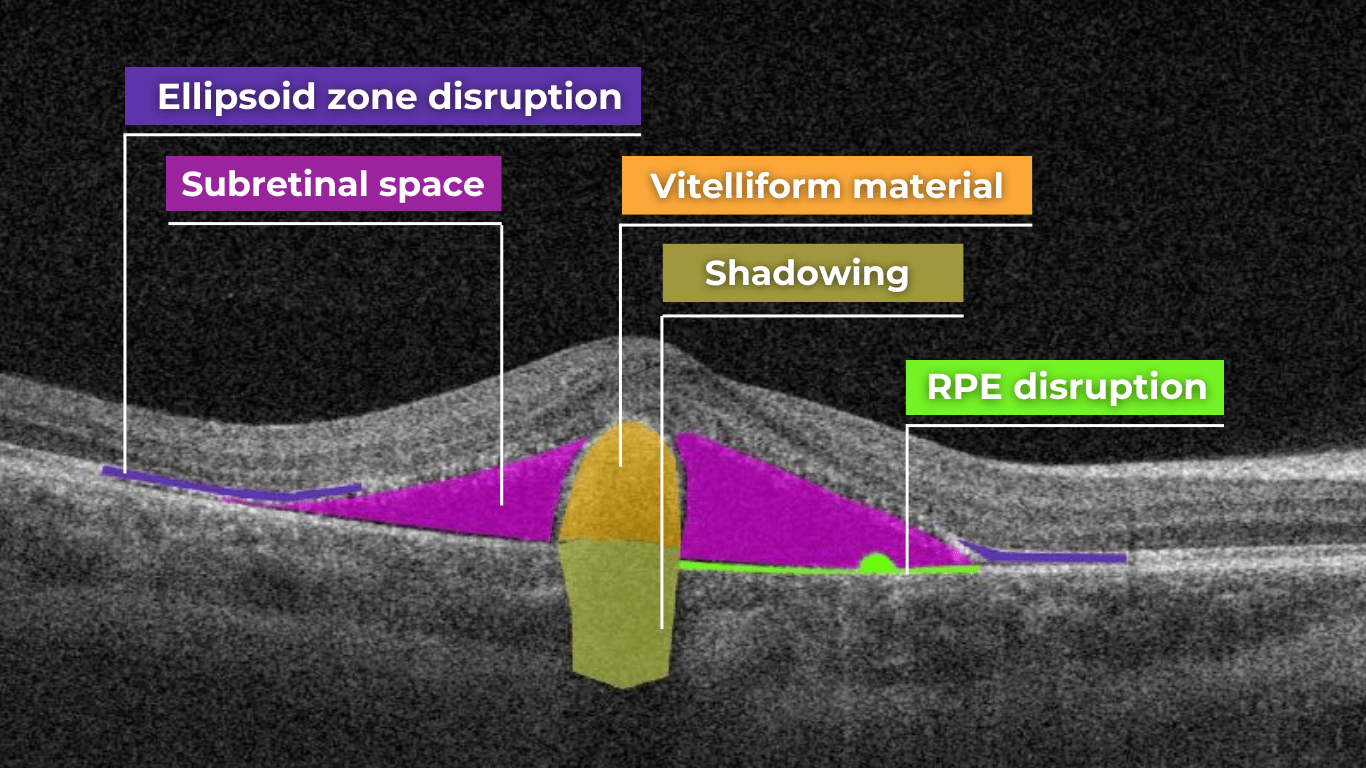
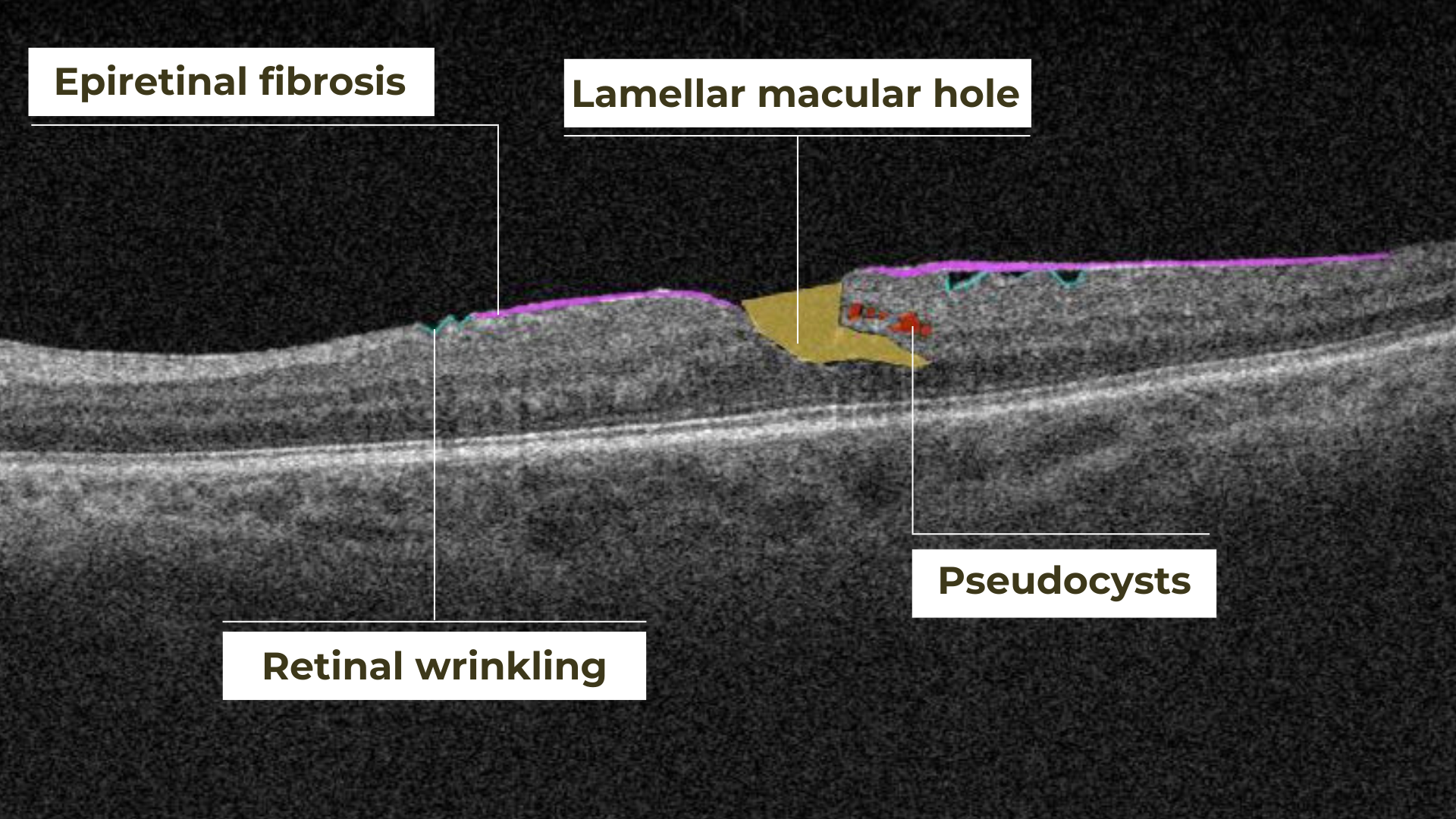
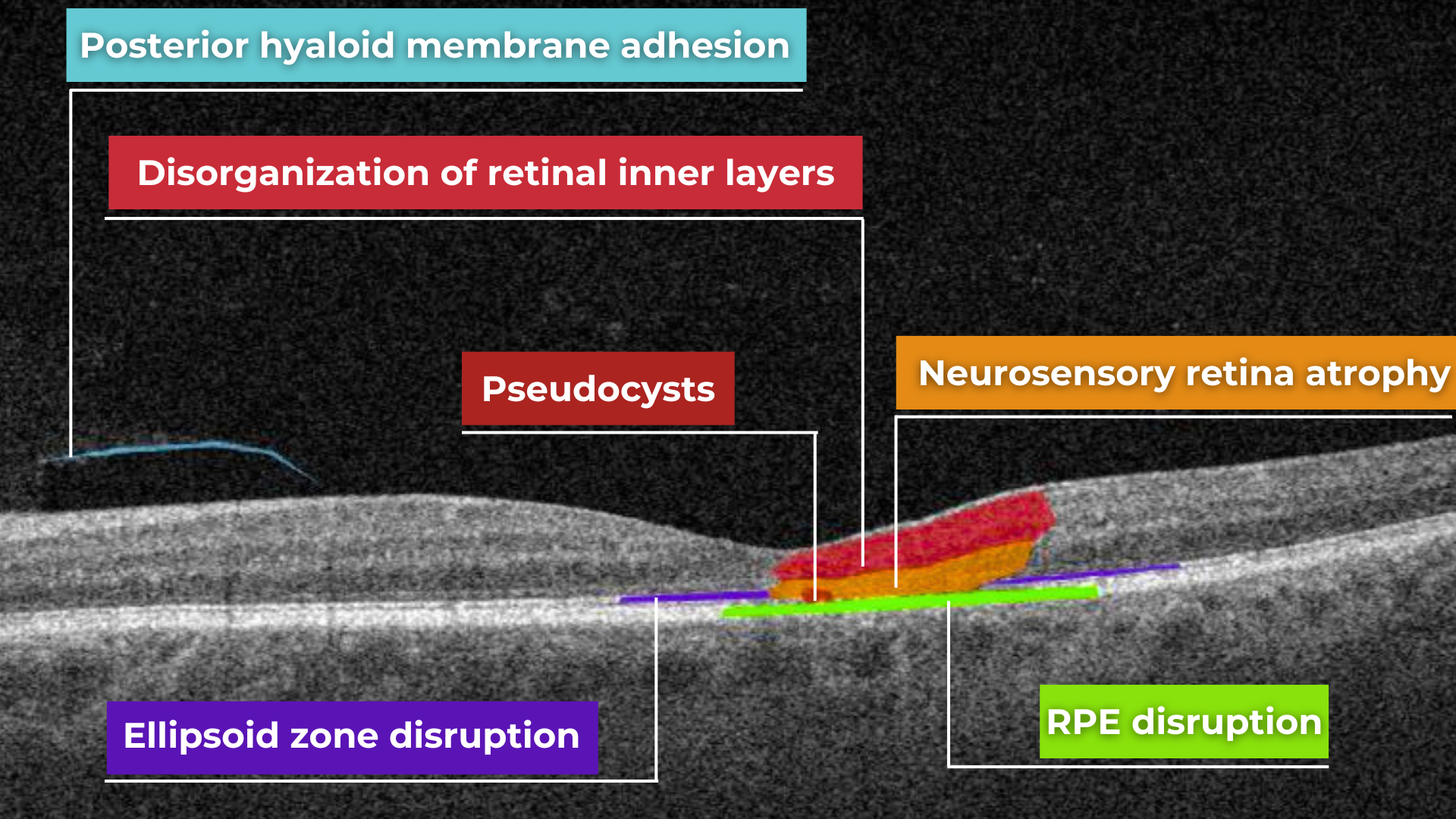
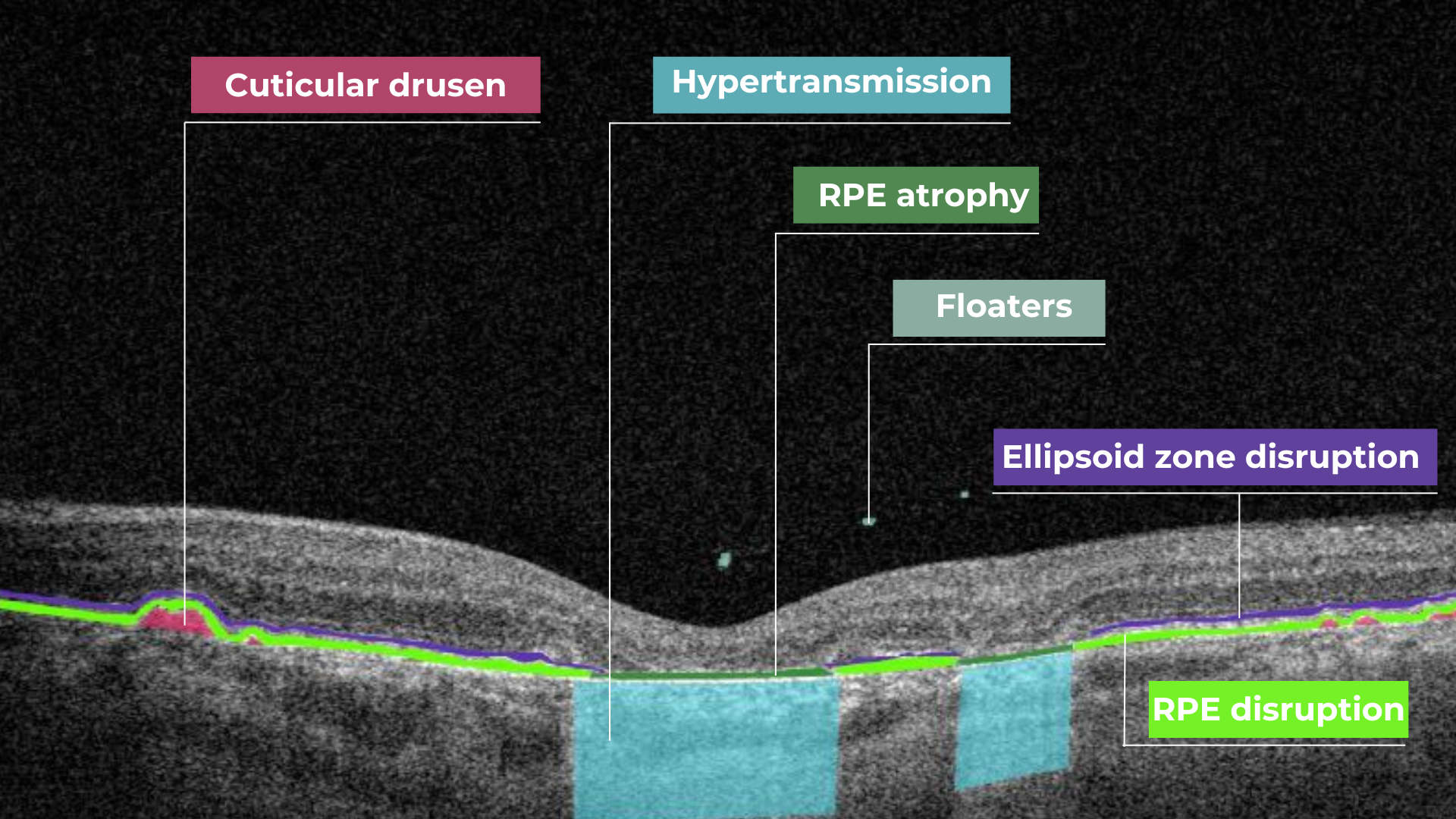 Hypertransmission on OCT
Hypertransmission on OCT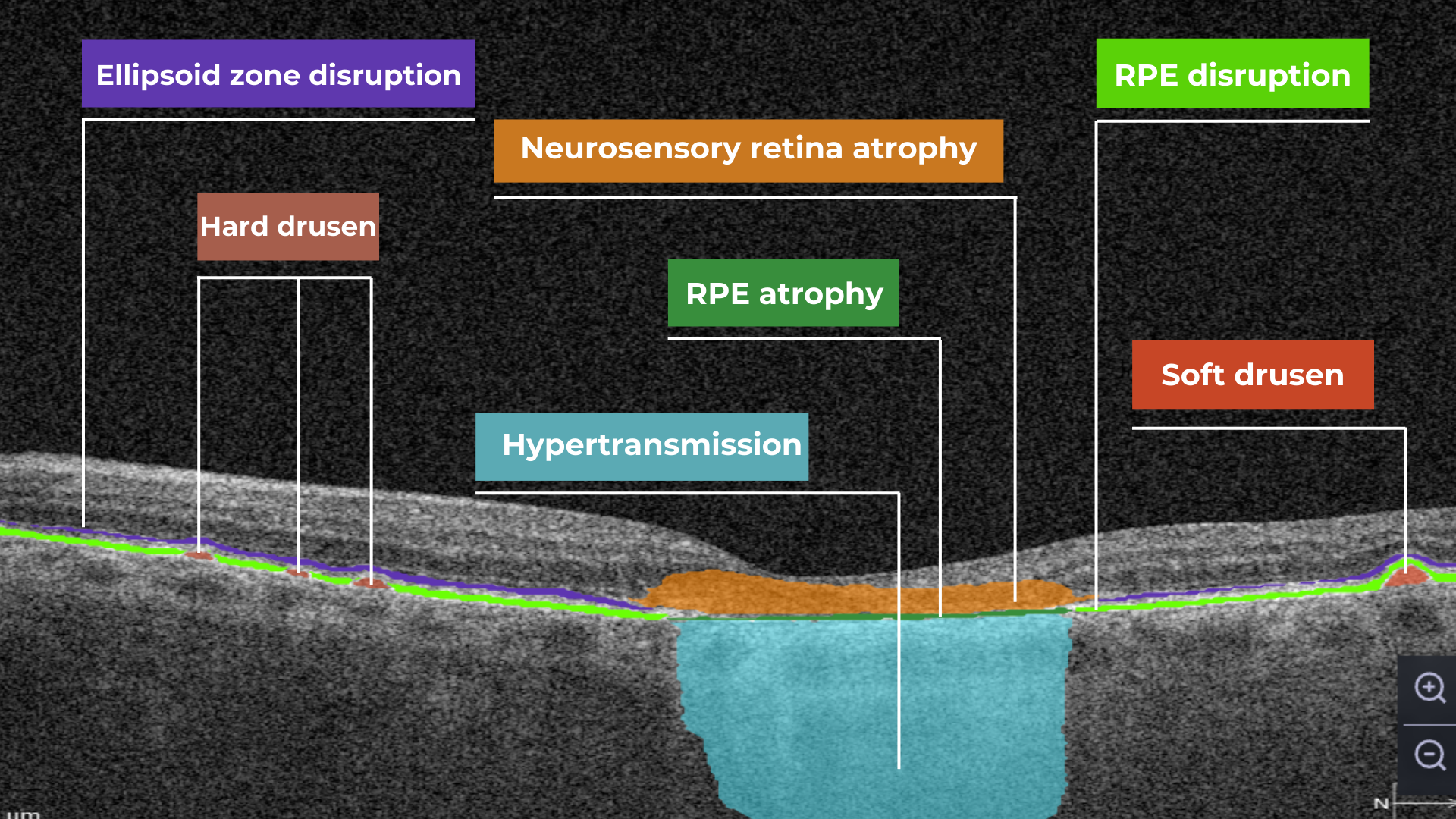
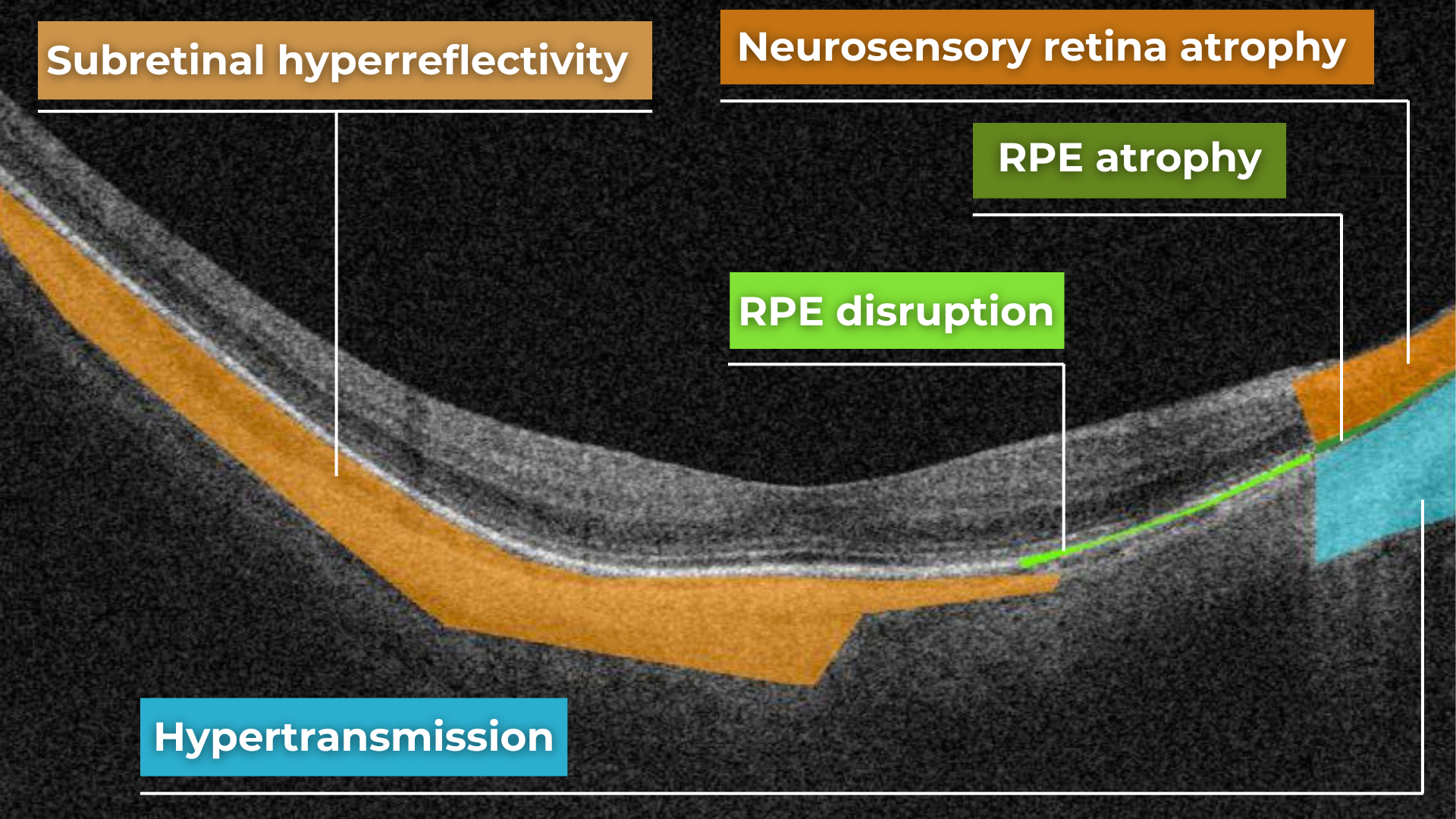
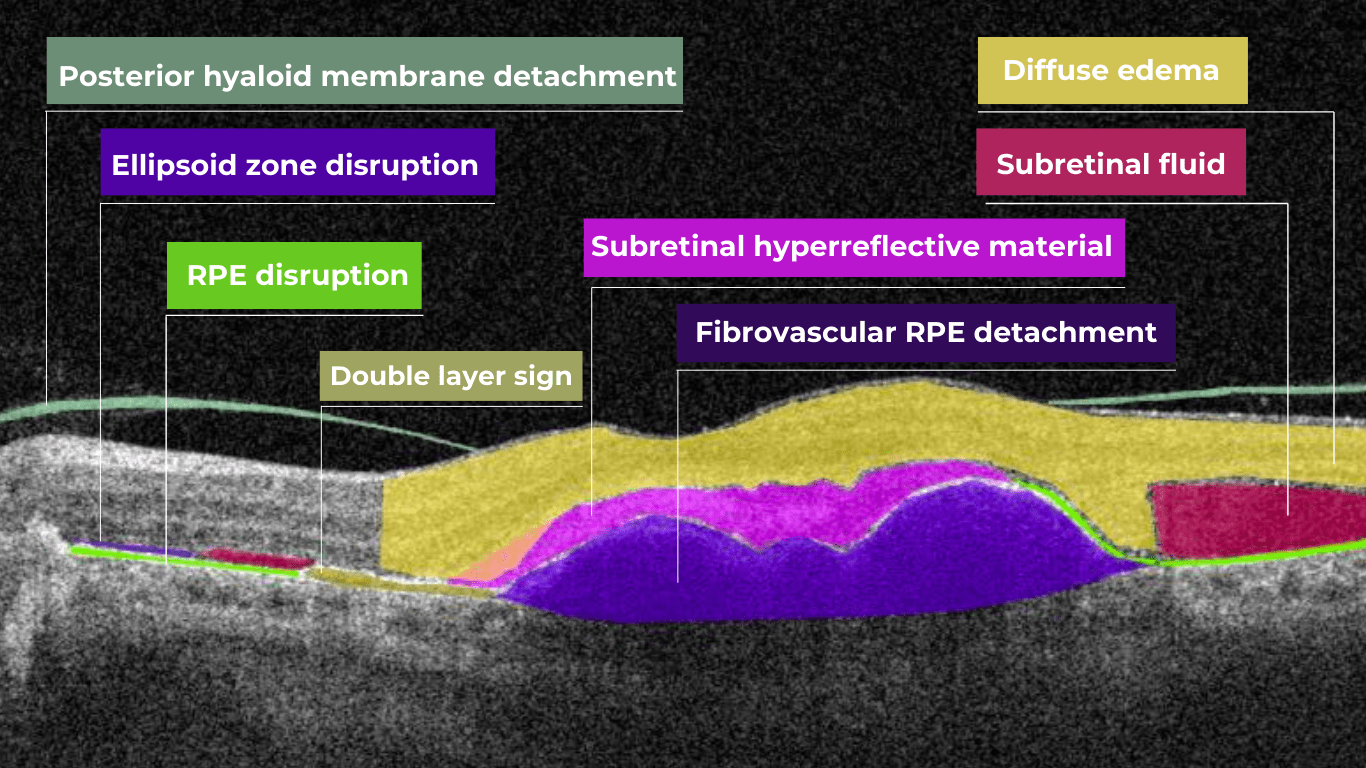
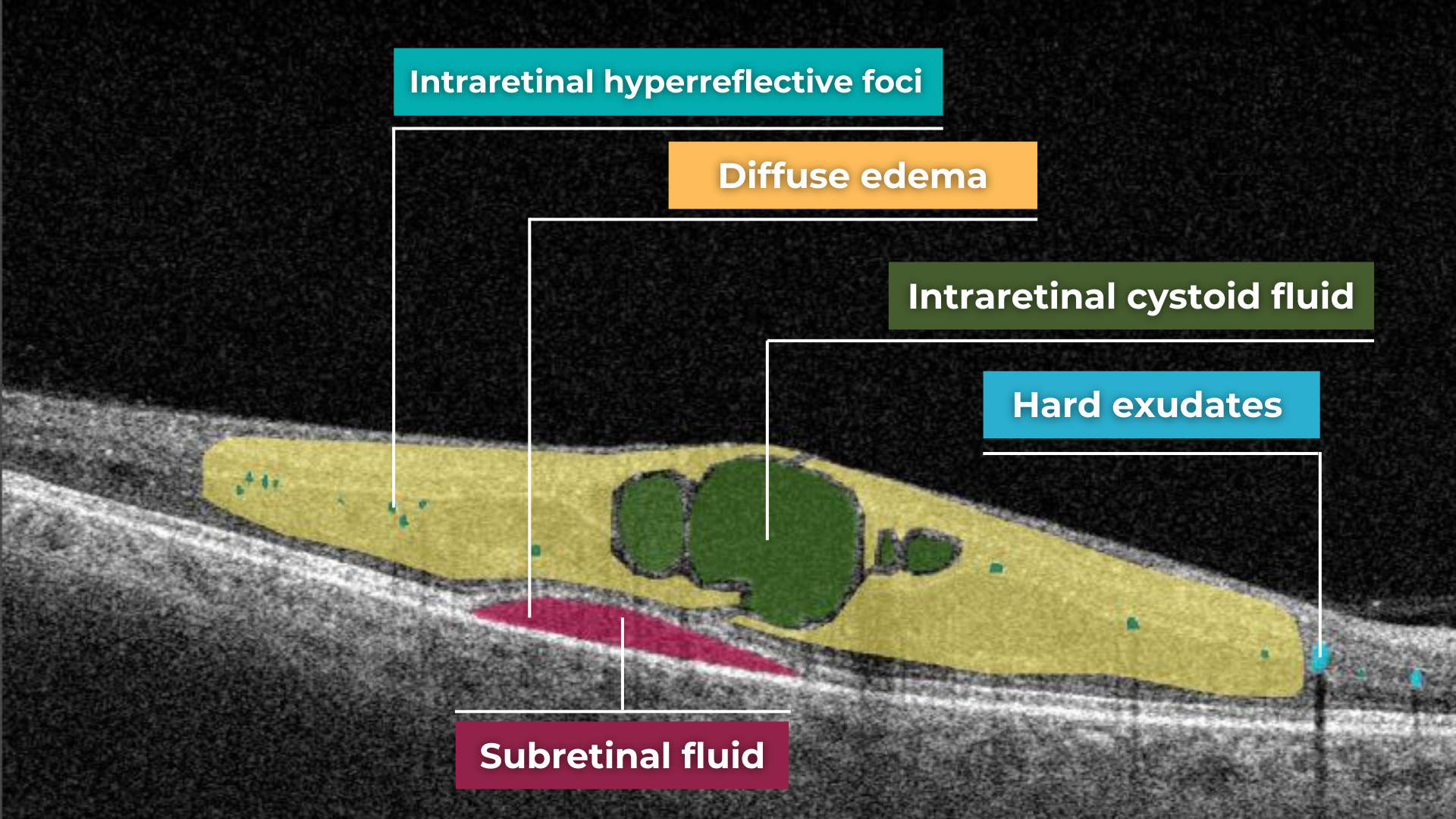













 AI in eye care can segment retinal structures to distinguish between normal retina scans and pathology on OCT, detect atrophic changes, and follow all alterations over time. It can even highlight rare inherited retinal dystrophies. For example,
AI in eye care can segment retinal structures to distinguish between normal retina scans and pathology on OCT, detect atrophic changes, and follow all alterations over time. It can even highlight rare inherited retinal dystrophies. For example,