OCT Management for Pharma
Reserach Use Only AI for retinal biomarkers Models for 30+ Pathologies & 40+ Biomarkers for Pharma Studies
Free Trial without CallsExplore Altris IMS
Explore the platform in a virtual demo or let us show it to you during the call
Free Trial

Virtual Demo

Altris IMS — Value for Pharmaceutical Professionals
✔️ Organize OCT data
✔️Access historical data
✔️Research Only AI Models
-
What ECPs think
-
"I am really happy with using Altris IMS in our research projects. And I was really impressed with the robustness of data."

-
 Alisdair Buchanan
Alisdair BuchananOptometry Owner
"Altris IMS has significantly enhanced the way we work with OCT scans"

-
 Dr. Sundeep Kheterpal
Dr. Sundeep KheterpalOphthalmic Surgeon
"We are very happy with the outstanding customer service and support."

-
 Jeff Sciberras
Jeff SciberrasOptometry Clinic Owner
"The introduction of OCT with Altris IMS has transformed my practice literally overnight. The integration was seamless and Altris customer support has been outstanding."







For Innovative Pharma
Support clinical reserach of 40+ retinal biomarkers and 30+ retinal pathologies on OCT with AI ROU models
Quantify and analyze 40+ retinal biomarkers & 30+ retinal pathologies to enable exploratory research on OCT. For Research Use Only.Not for use in diagnostic procedures.
Explore retinal biomarkers investigated in major research domains, including AMD, GA, DR, DME, RVO, and others.
Automate research cohort characterization and retrospective analysis. For Research Use Only. Not for use in diagnostic procedures.
Gather analytics on eligible patients using AI for retinal biomarkers RUO models for 40+ retinal biomarkers in relation to 30+ retina conditions. For Research Use Only. Not for use in diagnostic procedures.
Power your research on 40+ retinal biomarkers in relation to 30+ retina conditions. For Research Use Only. Not for use in diagnostic procedures.
Quantify and analyze 40+ retinal biomarkers relevant to the research of 30+ retinal conditions For Research Use Only. Not for use in diagnostic procedures.
Explore 40+ retinal biomarkers studied across major retinal research areas (Dry & Wet AMD, GA, DR, DME, RVO). For Research Use Only. Not for use in diagnostic procedures.
Gather analytics on eligible patients using AI RUO models for 40+ retinal biomarkers in relation to 30+ retina conditions. For Research Use Only. Not for use in diagnostic procedures.
Results in numbers
Our company was created in 2017 and we achieved many milestones since then
Let's talk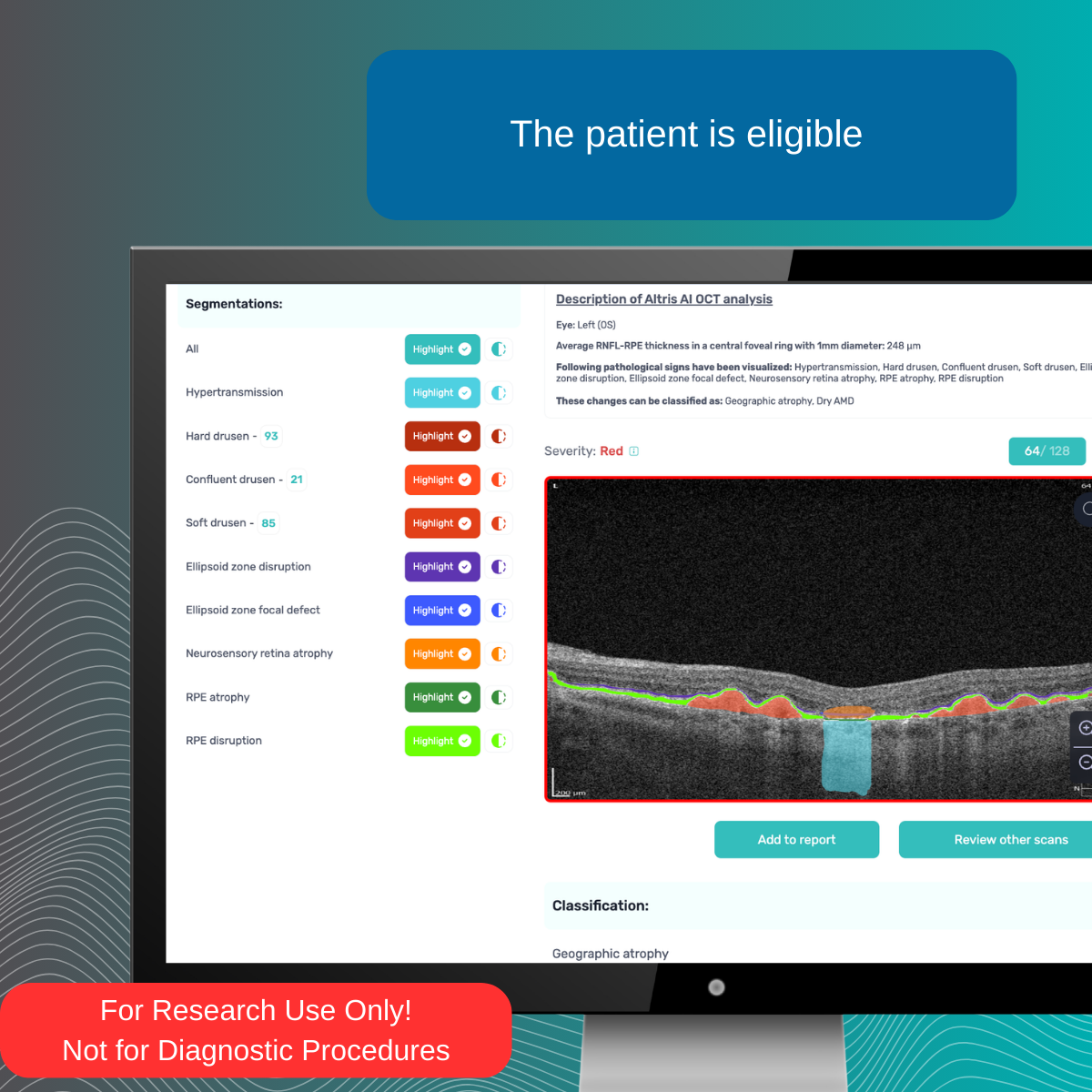
For Commercial Specialists
Support identification of patient cohorts based on OCT imaging characteristics, aligned with approved indications
Offer clear visualization and analysis of OCT imaging features across patient populations relevant to emerging treatment types
Enable retrospective analysis of OCT data from multiple manufacturers to support population-level insights
Provide access to aggregated real-world evidence (RWE) imaging data and longitudinal biomarker observations for analytical purposes
Support clinical workflows by assisting clinicians in the review, organization, and visualization of OCT imaging data

For Clinical Researchers
Assist research teams in identifying potential study populations through OCT image analysis, in accordance with protocol-defined criteria
Support retrospective analysis of large volumes of OCT data from multiple manufacturers
Enable objective comparison of imaging-derived biomarkers across pre- and post-study timepoints for research and observational analysis
Configure study-specific filtering criteria to explore and query large OCT imaging datasets
Research
Running clinical studies shouldn’t be slowed down by manual processes or fragmented data
Contact Us- Support analysis of historical OCT data
- Consistent OCT image research
- Support of protocol-defined patient review
- OCT review consistency and reproducibility
- Vendor-neutral OCT data access
- Support of longitudinal imaging review
- Quantitative imaging outputs
Commercialization
Supporting transparency and data-driven insight across the drug commercialization process
Contact Us- Power clinical workflows by assisting users in reviewing and organizing OCT imaging data
- Support understanding of patient populations
- Deliver transparent, aggregated insights on patient cohorts
- Enable access to de-identified, observational real-world data
- Support educational initiatives for ECPs
- Provide data-informed analytics to support pharmaceutical commercialization activities







Medical Director