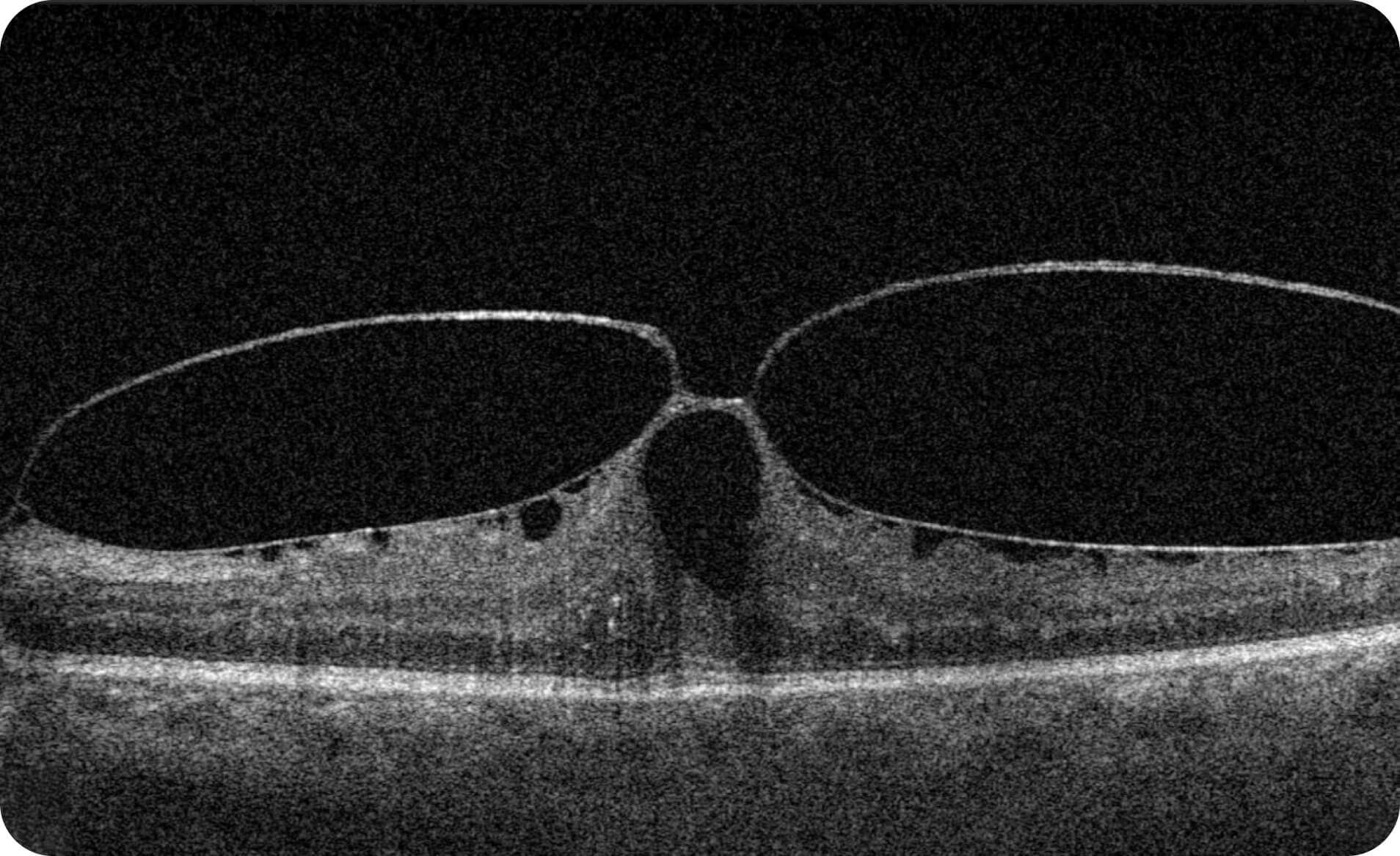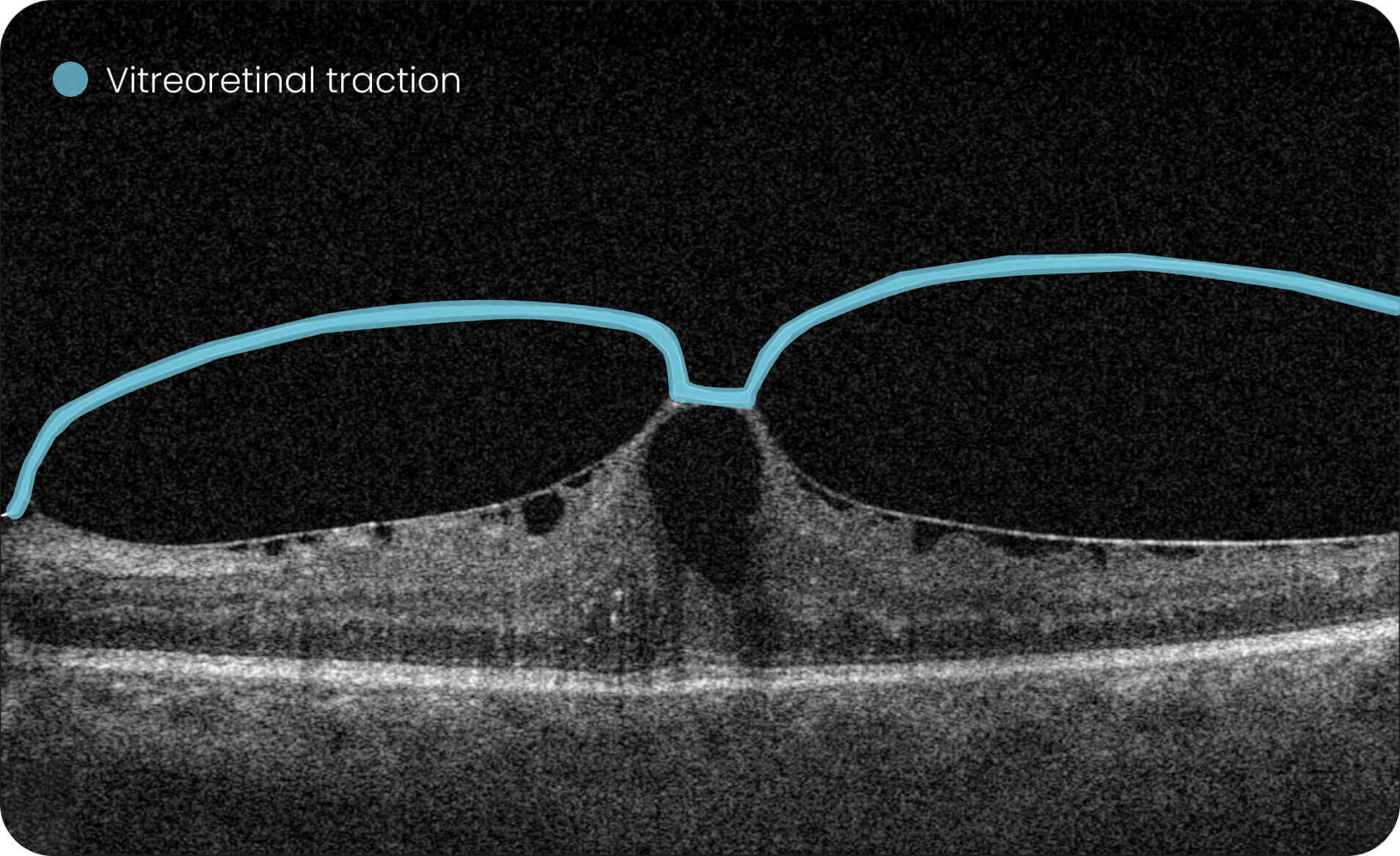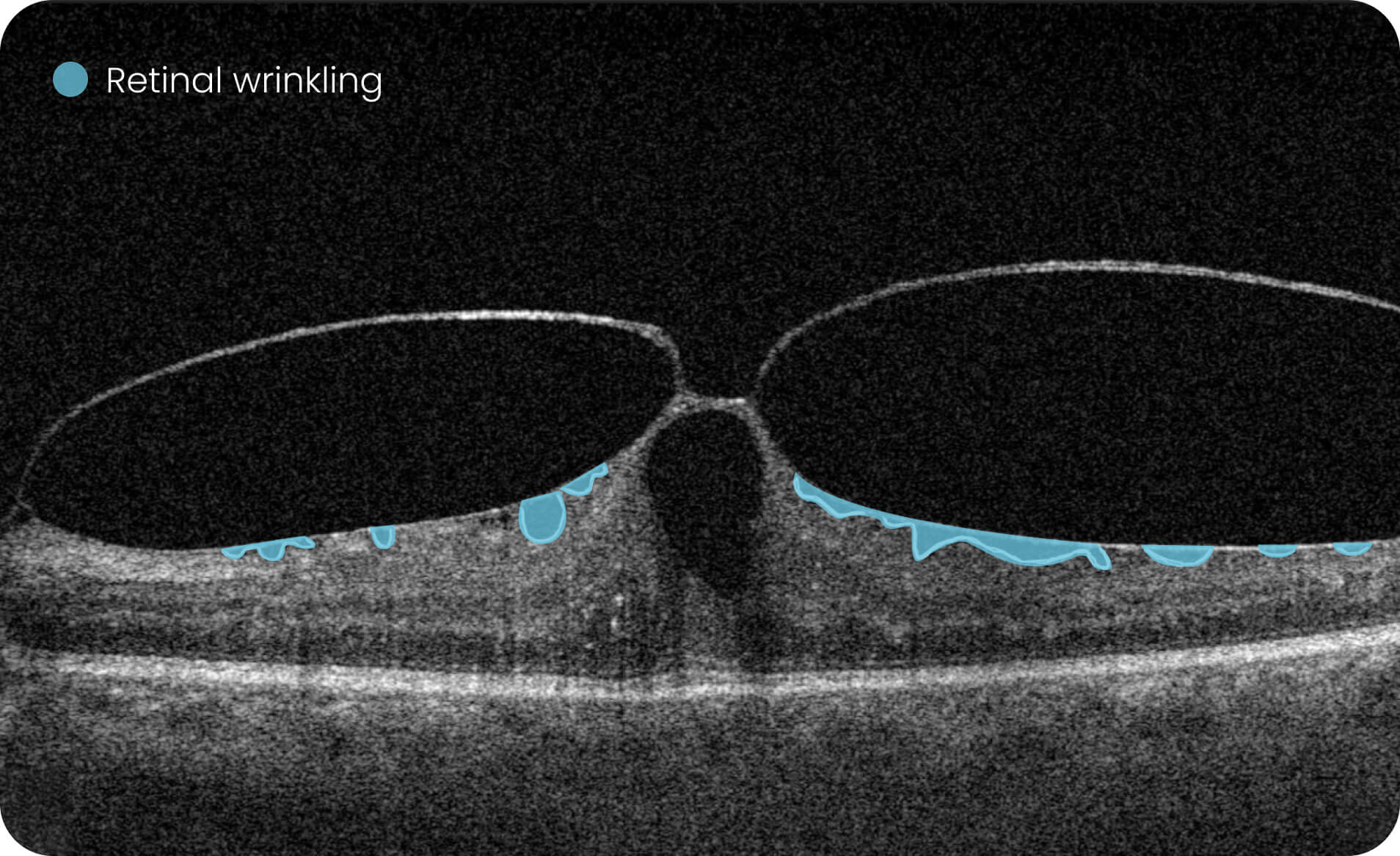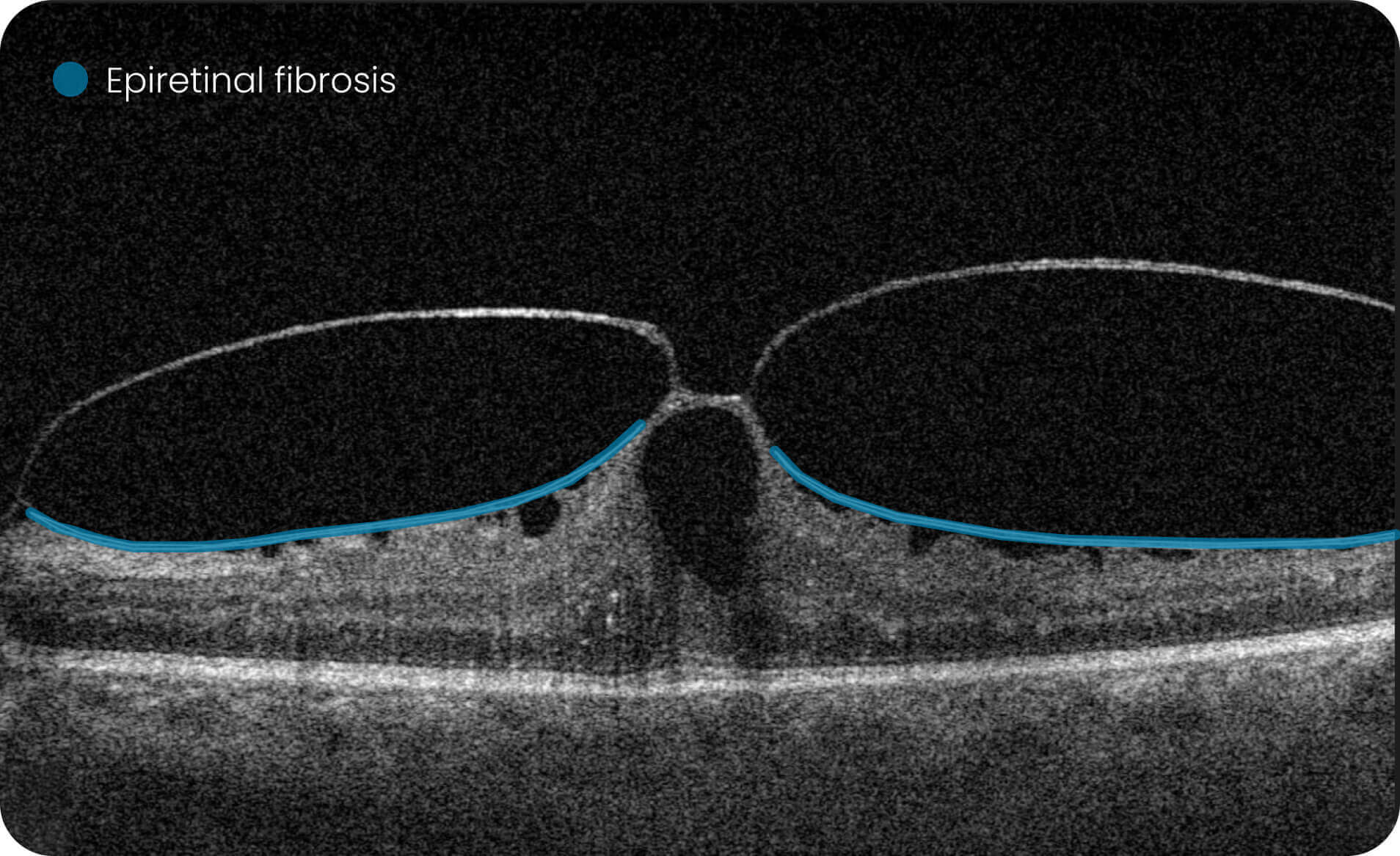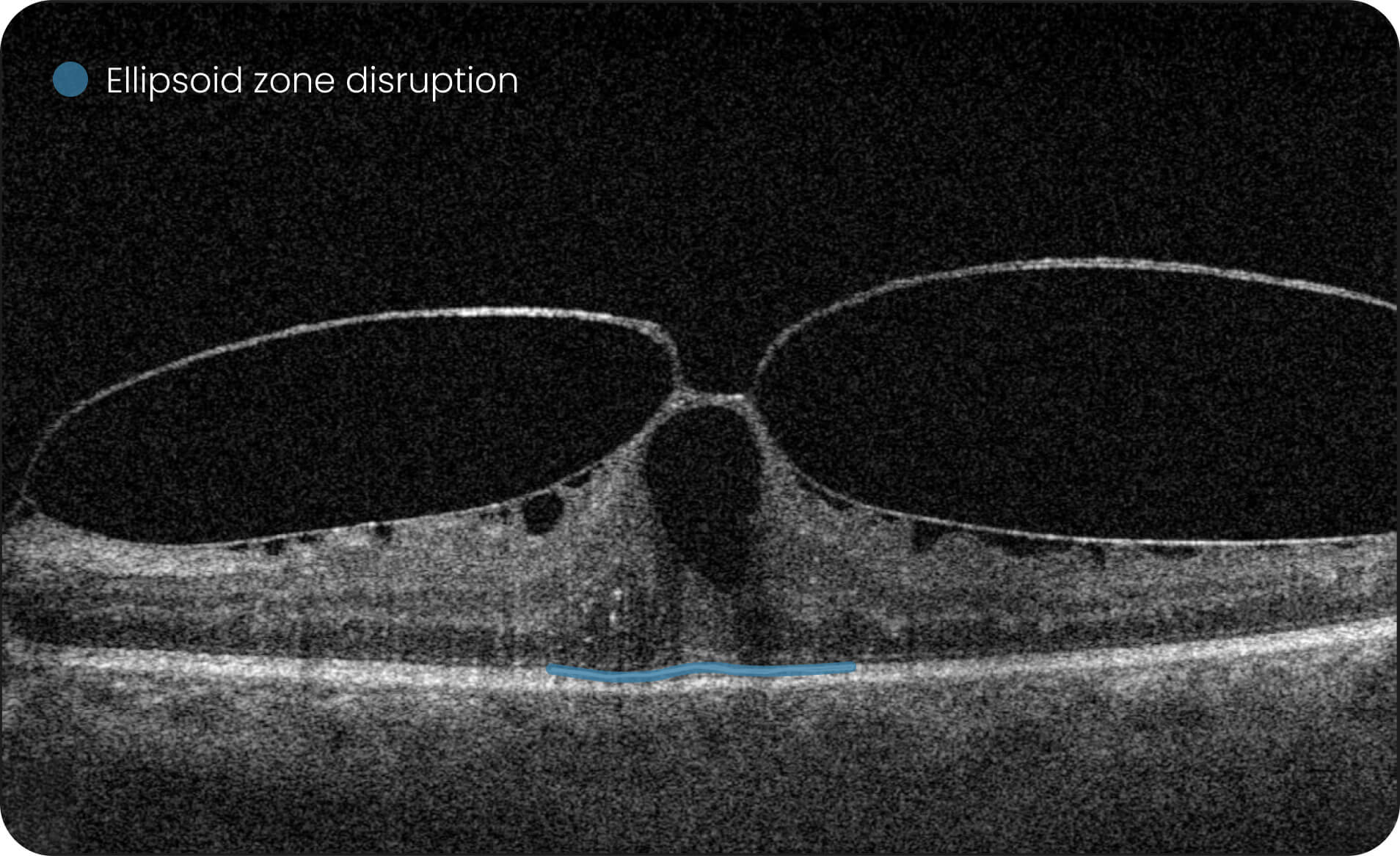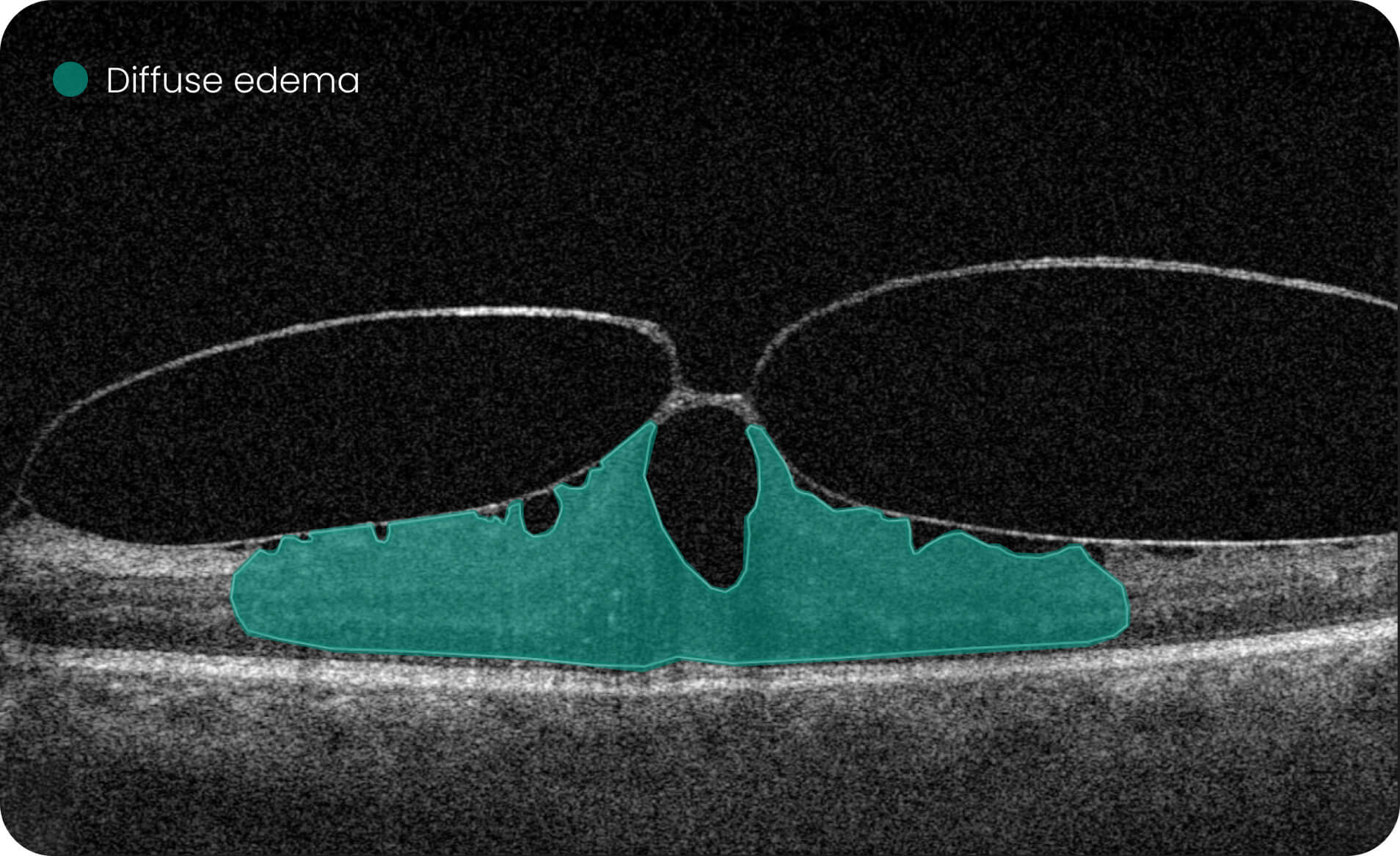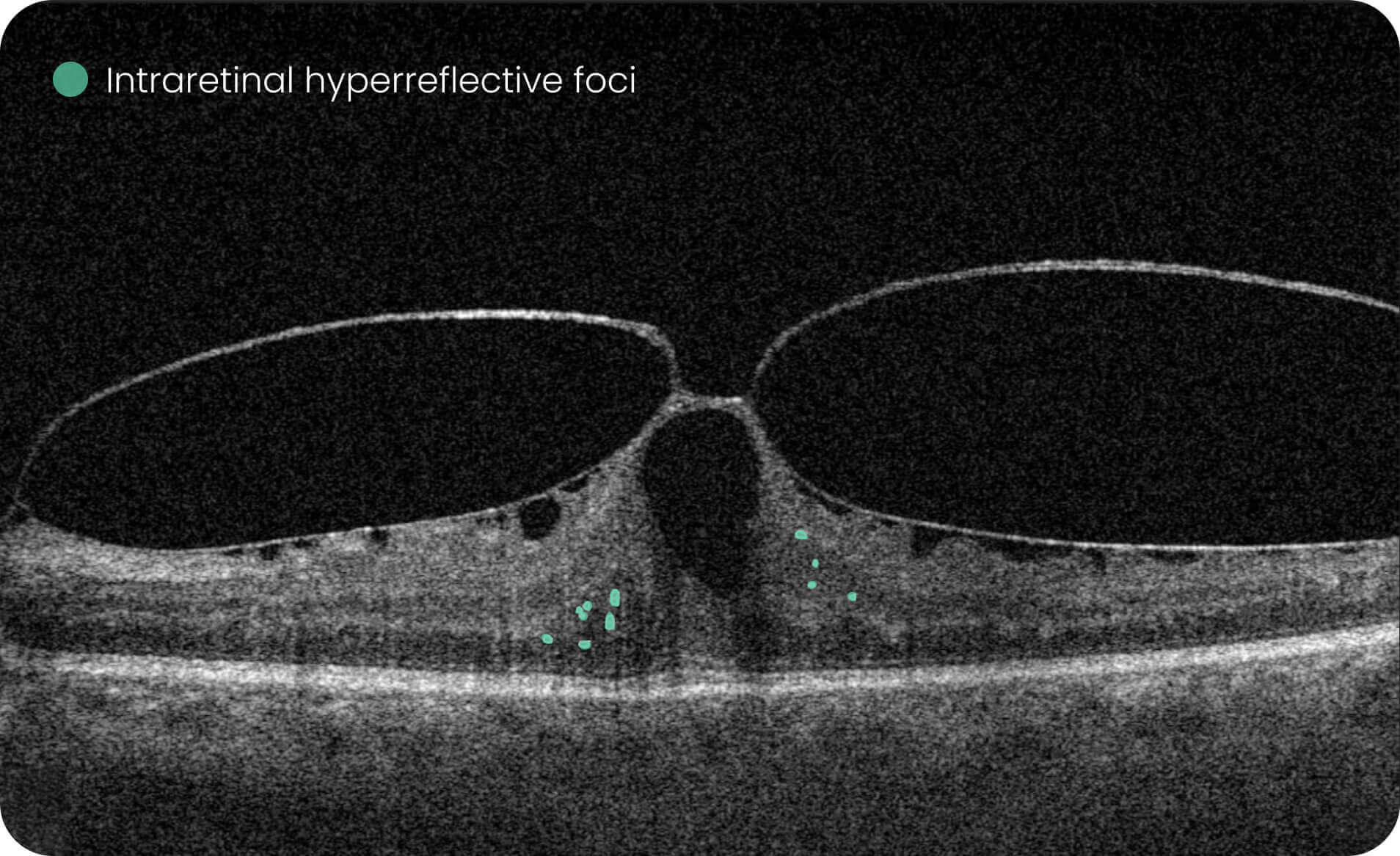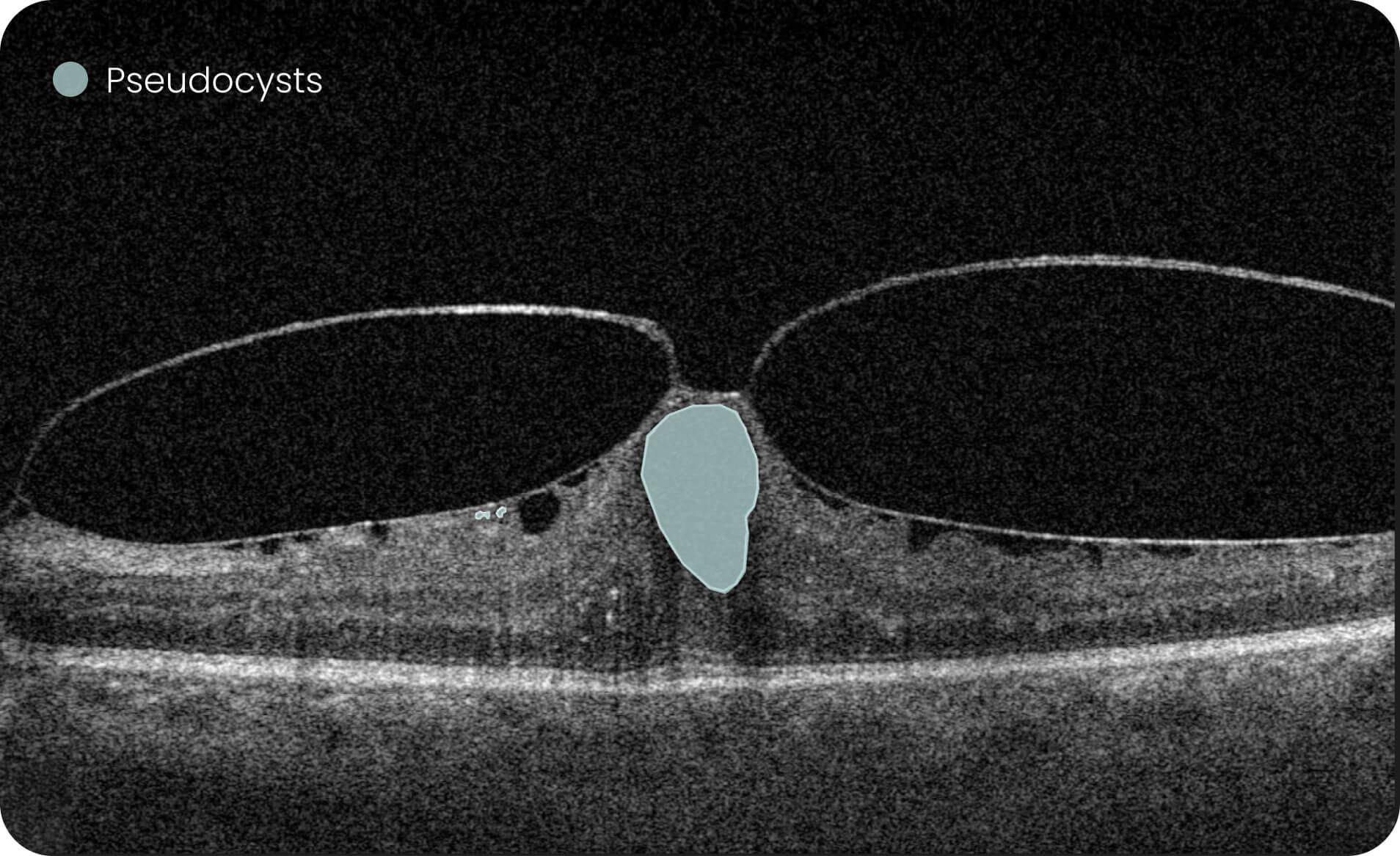
Altris IMS is an FDA-cleared (510(k)) image management system for OCT. Intended to support research workflows by enabling:
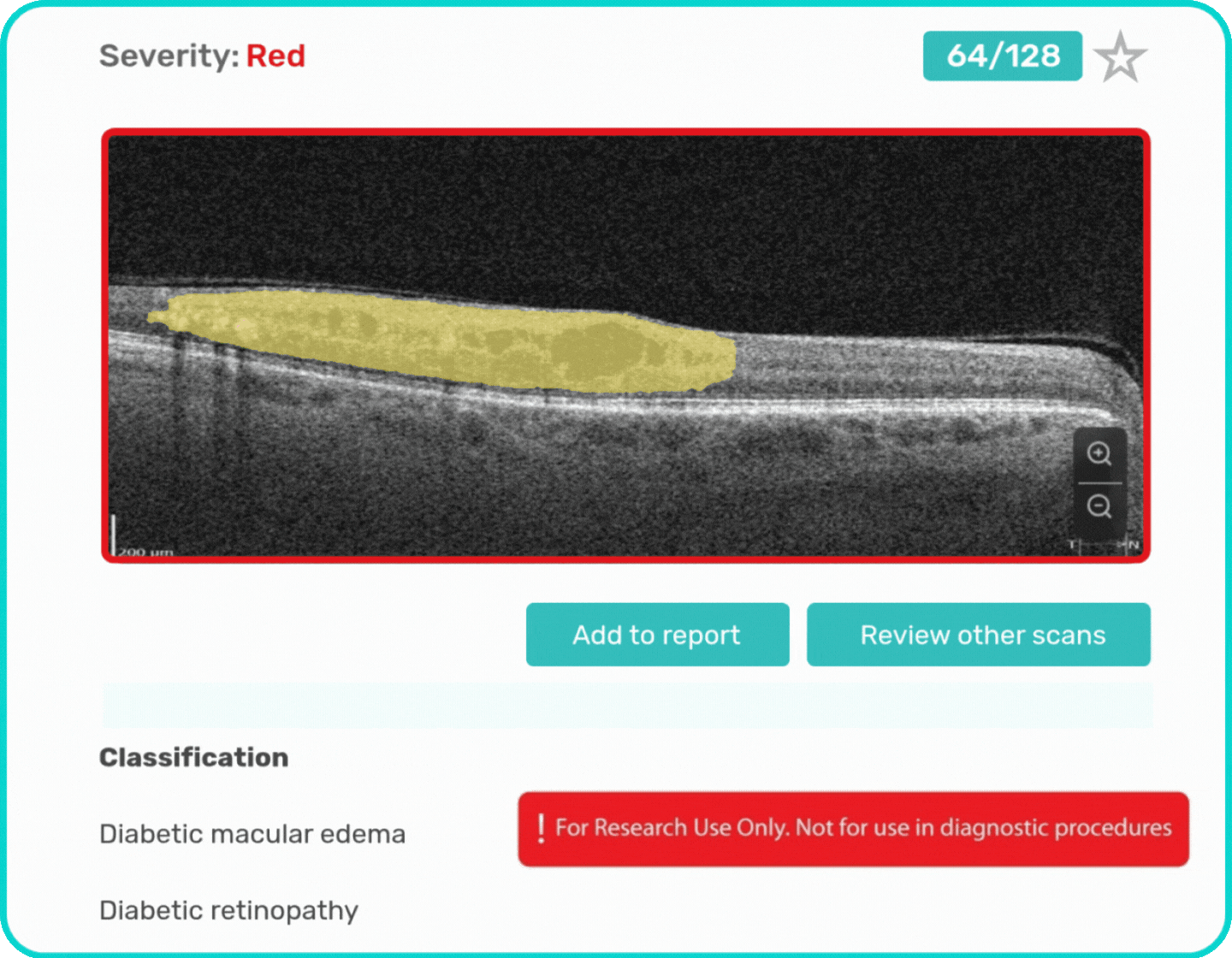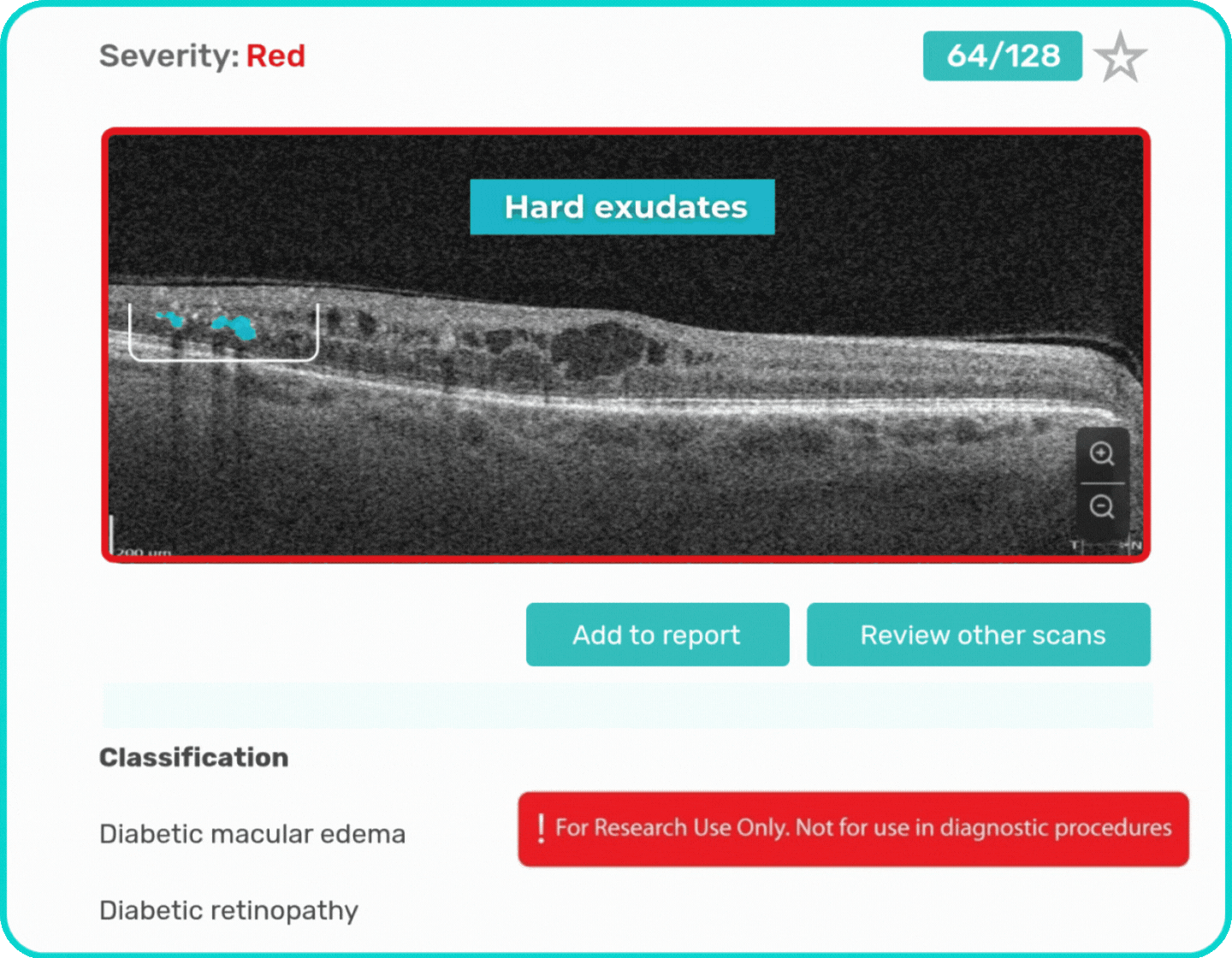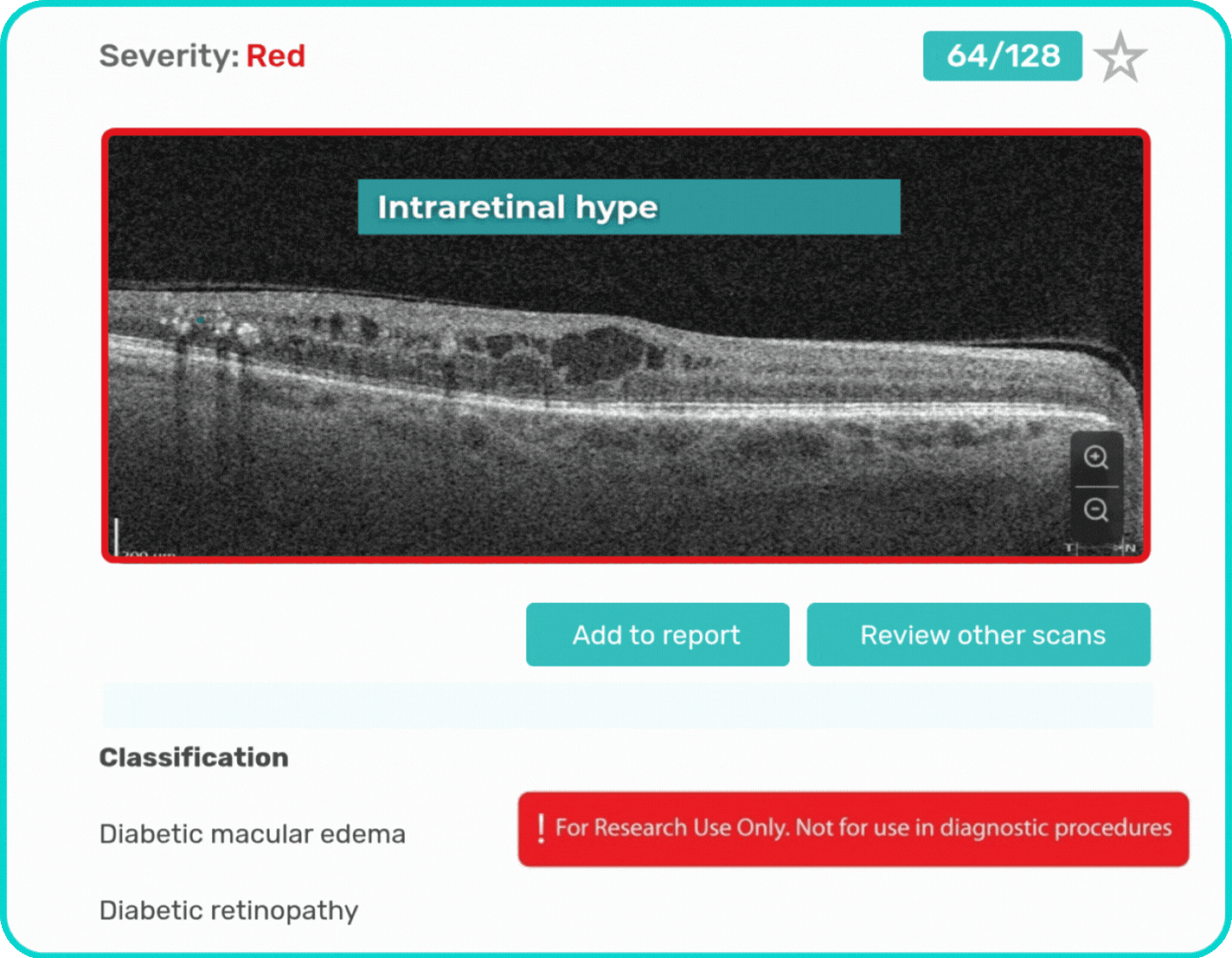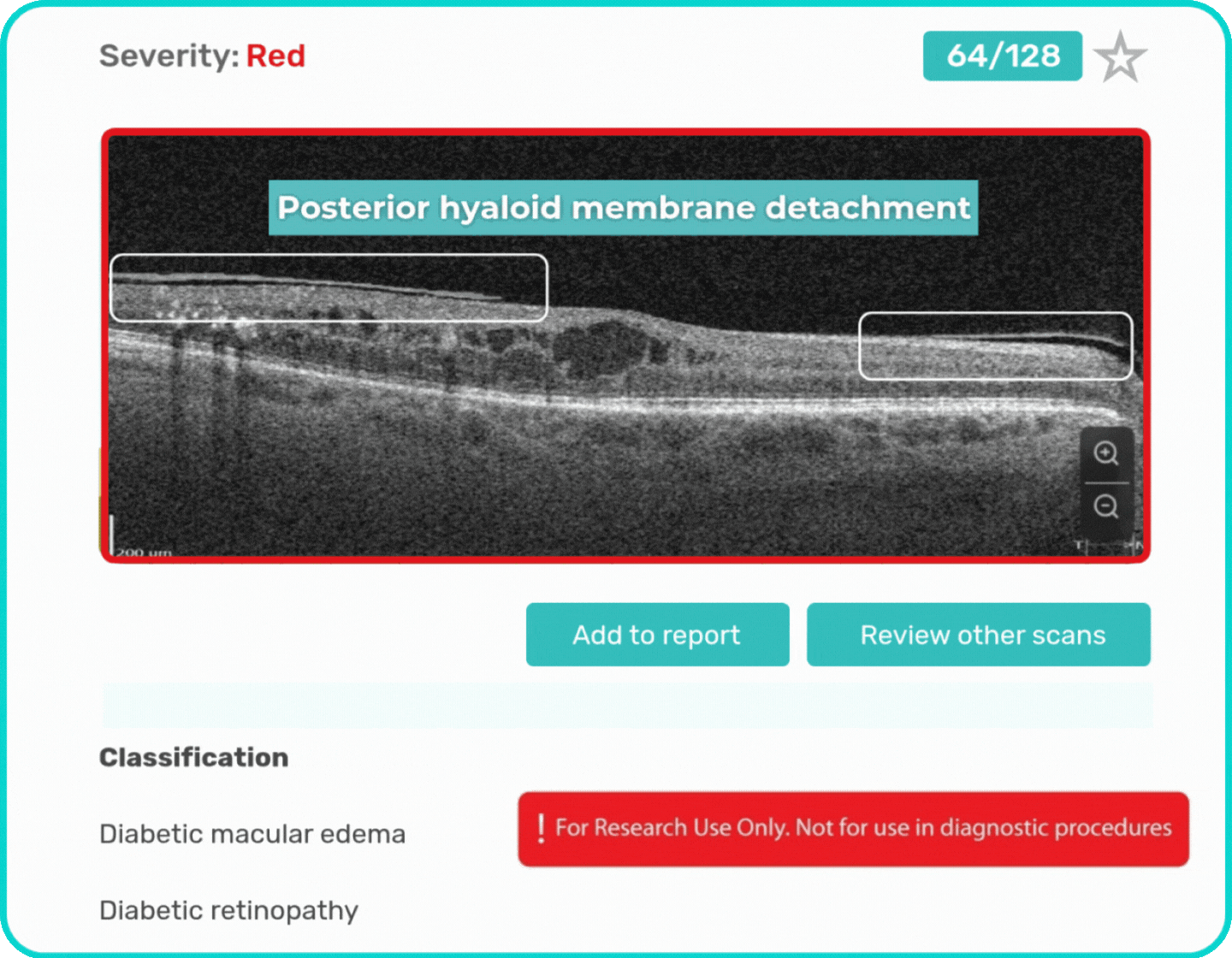
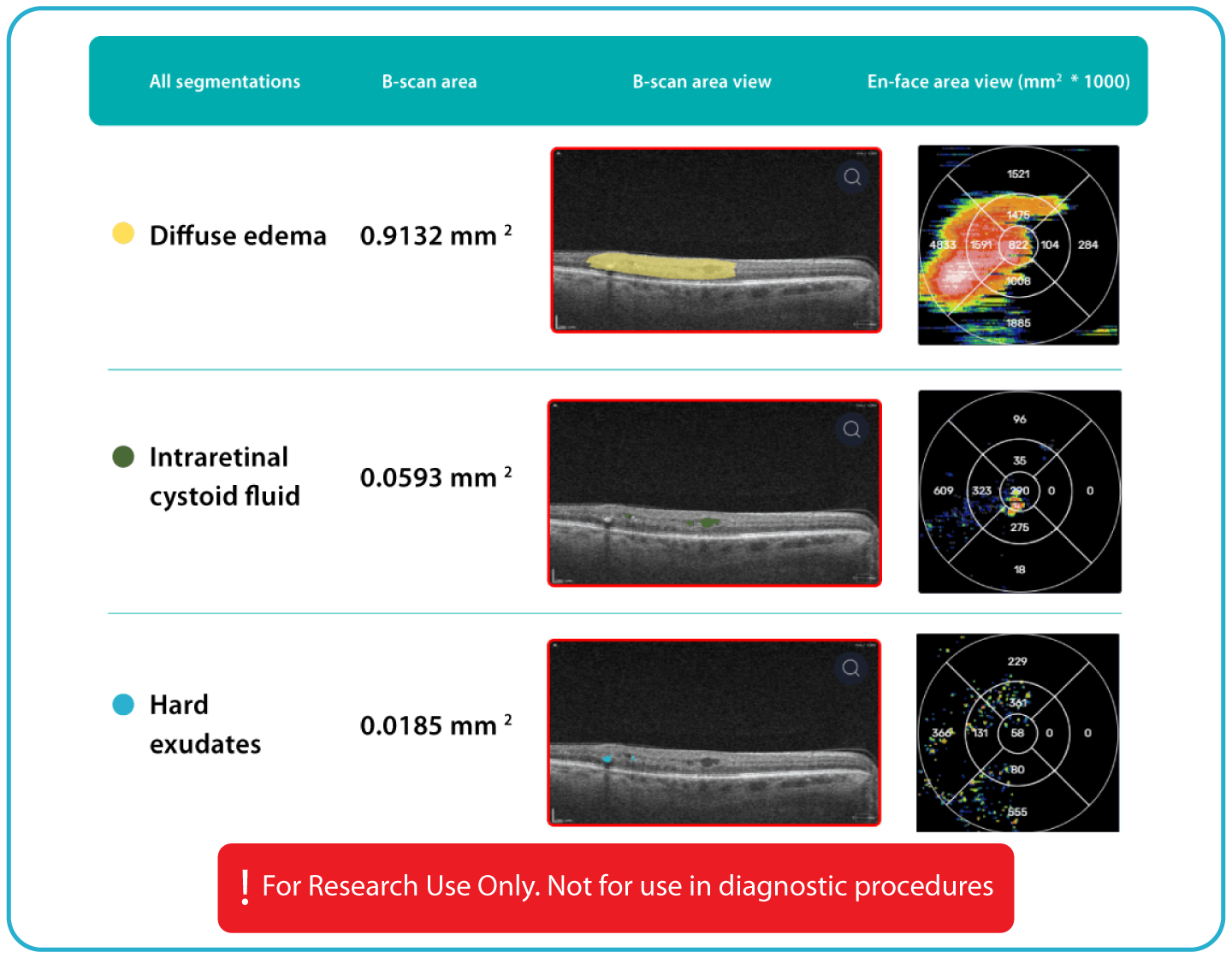
- Identification and visualization of OCT imaging features commonly associated with DR, including diffusee edema, subretinal fluid, vitelliform material, intraretinal hyperreflective foci, as well as less frequently described features such as epiretinal fibrosis and retinal surface irregularities.
- Quantitative measurements of selected DR-related imaging features, with spatial information presented on ETDRS-style maps and B-scan visualizations.
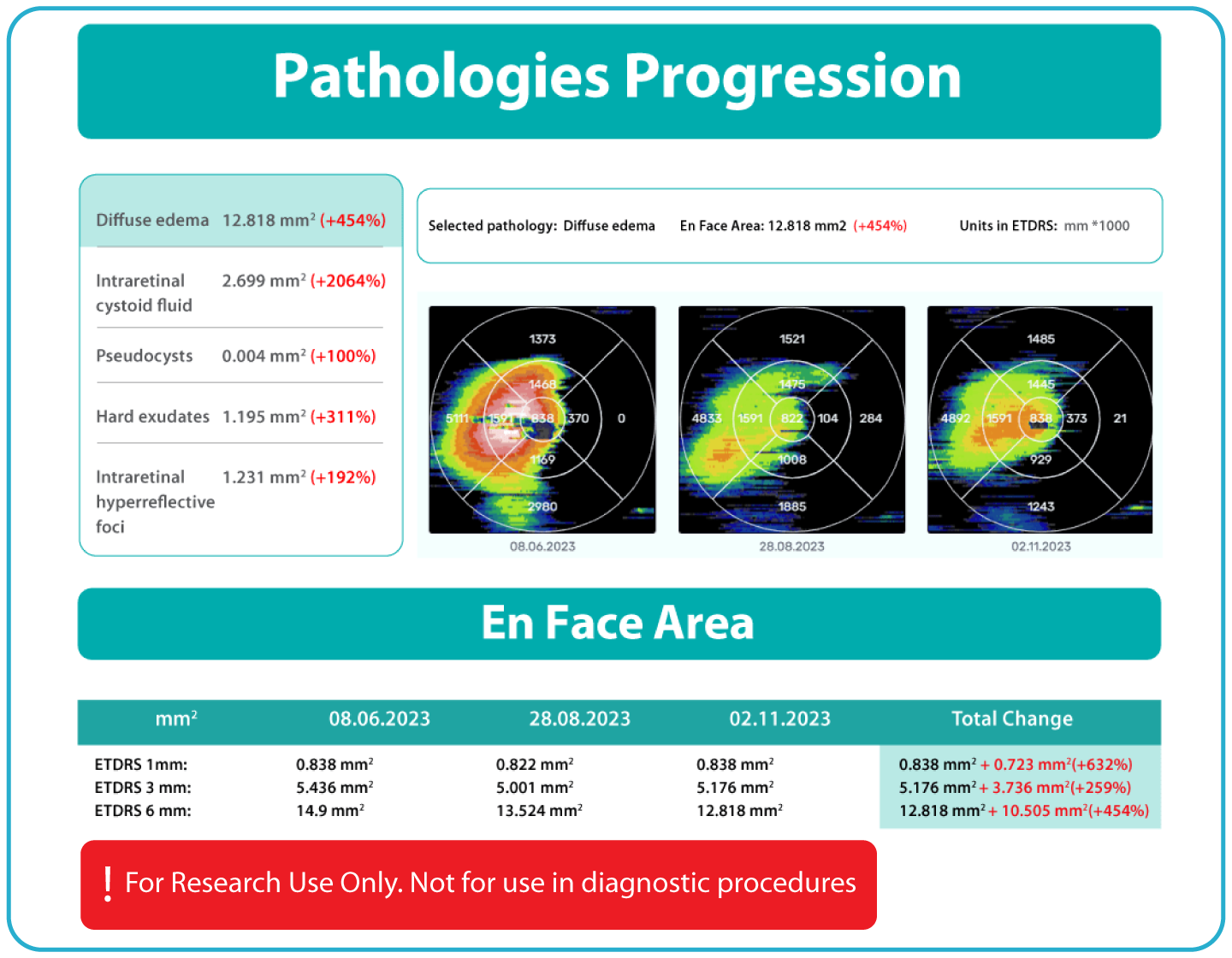
- Automated comparisons of imaging data across multiple visits can be viewed through percentage-based displays, maps, and graphs.
For research use only. Not for diagnostic or clinical decision-making.






- Platform overview
Only practical features for eye care specialists and clinical research
The security of patients’ data is our top priority: we are GDPR compliant, all data is encrypted, CE-certified, and FDA-cleared (510k) as an IMS system.
The system provides tools for research-focused review of longitudinal OCT data for US retina studies to observe changes in image-derived features associated with diabetic retinopathy (DR). Thus, the Pathology Progression module offers quantitative, image-derived measurements of selected DR-related features, including fine-scale changes, to support research analysis.
- Automated identification and visualization of imaging features associated with DR to support efficient image-review workflows.
- Objective, quantitative measurements of DR-related imaging features for use in research.
- Visualization of feature changes over time, supported by maps and graphical displays.
- Longitudinal image-based data to assist with analysis and stratification activities in research settings. For research use only. Not for diagnostic or clinical decision-making.
Label all the biomarkers of DR with color-coding
Explore DR biomarkers in terms of area, volume
How Altris IMS works?
What makes Altris IMS effective for Diabetic Retinopathy research?
Altris IMS is a web-based platform developed by professionals with expertise in retinal imaging. The system is built using a large collection of OCT scans, thousands of which have been manually annotated for research and development purposes. These data support AI-based image-analysis tools that can:
For research use only. Not for diagnostic or clinical decision-making.
Formats
DICOM format will help you to extract maximum information. However, the system works with all
data formats, such as jpg, and png
OCT equipment
Altris IMS is vendor-neutral. We work with the OCT data from 8 OCT manufacturers
OCT reports
We create comprehensible OCT reports for patients and eye care specialists based on historical research
For Pharma
For innovative approach in pharma
Contact usWhat you get:
- Centralized OCT Data Management
- Historical Data Analysis
- Vendor-neutral analysis of OCT scans (8 manufacturers)
- Data Security & Compliance
- Additional capabilities for research
- 40+retinal biomarkers studied in research across 30+ retinal conditions. For Research Use Only. Not for diagnostic procedures.
- Quantitative exploration of 40+biomarkers for Research Use Only. Not for diagnostic procedures.
- Reports
For Eye Care
IMS for Ophthalmology and Optometry
Contact usWhat you get:
- Centralized OCT Data Management
- Vendor-Neutral OCT Compatibility (8 manufacturers)
- Secure & Compliant Data Environment
- Historical OCT Data Analysis
- Seamless Clinical Workflow Integration
- 40+retinal biomarkers studied in research across 30+ retinal conditions. For Research Use Only. Not for diagnostic procedures.
- Quantitative exploration of 40+biomarkers for Research Use Only. Not for diagnostic procedures.