IMS for Optometry
Reserach Use Only AI in Optometry Models for 30+ Retinal Pathologies & 40+ Biomarkers
Free Trial without Calls
Explore Altris IMS
Explore the platform in a virtual demo or let us show it to you during the call
Free Trial

Virtual Demo

Altris IMS — Value for Organizing OCT Data for Optometry
✔️ Organize OCT data
✔️Access historical data
✔️Research Only AI Models
-
What ECPs think
-
"I am really happy with using Altris IMS in our research projects. And I was really impressed with the robustness of data."

-
 Alisdair Buchanan
Alisdair BuchananOptometry Owner
"Altris IMS has significantly enhanced the way we work with OCT scans"

-
 Dr. Sundeep Kheterpal
Dr. Sundeep KheterpalOphthalmic Surgeon
"We are very happy with the outstanding customer service and support."

-
 Jeff Sciberras
Jeff SciberrasOptometry Clinic Owner
"The introduction of OCT with Altris IMS has transformed my practice literally overnight. The integration was seamless and Altris customer support has been outstanding."







Enhancing the OCT workflow
Vendor-neutrality: work with data from 9 OCT manufacturers in Altris IMS.
Organize historical OCT data.
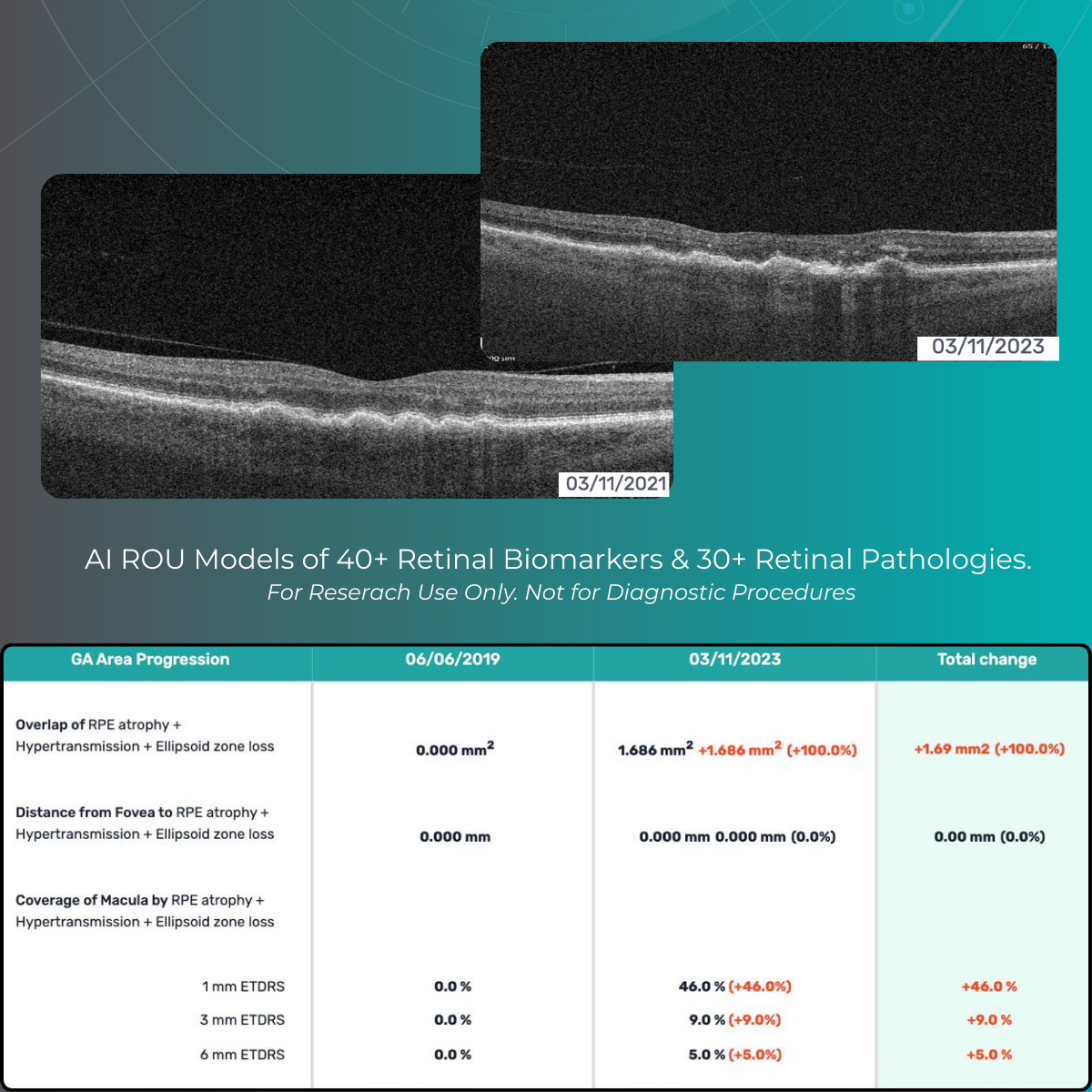
Power your research on 40+ retinal biomarkers in relation to 30+ retina conditions. For Research Use Only. Not for use in diagnostic procedures.
Analyze 40+ retina biomarkers — supporting research on 30+ retinal pathologies. For Research Use Only. Not for use in diagnostic procedures.
Explore 40+ retinal biomarkers studied across major retinal research areas (e.g., AMD subtypes and DR). For Research Use Only.Not for use in diagnostic procedures.
Vendor-neutral OCT data support — enables access to historical OCT data from up to 9 major OCT manufacturers within Altris IMS.
Structured organization of historical OCT scans to support retrospective review and research workflows.
Research-focused analysis of 40+ retinal imaging biomarkers studied across 30+ retinal conditions, including major retinal research areas such as AMD subtypes and diabetic retinopathy. For Research Use Only (RUO). Not for use in diagnostic, screening, or clinical decision-making procedures.
Quantitative biomarker outputs are intended to support exploratory analysis, academic research, and internal investigations. For Research Use Only (RUO). Not for use in diagnostic, screening, or clinical decision-making procedures.
Results in numbers
Our company was created in 2017 and we achieved many milestones since then
Let's talk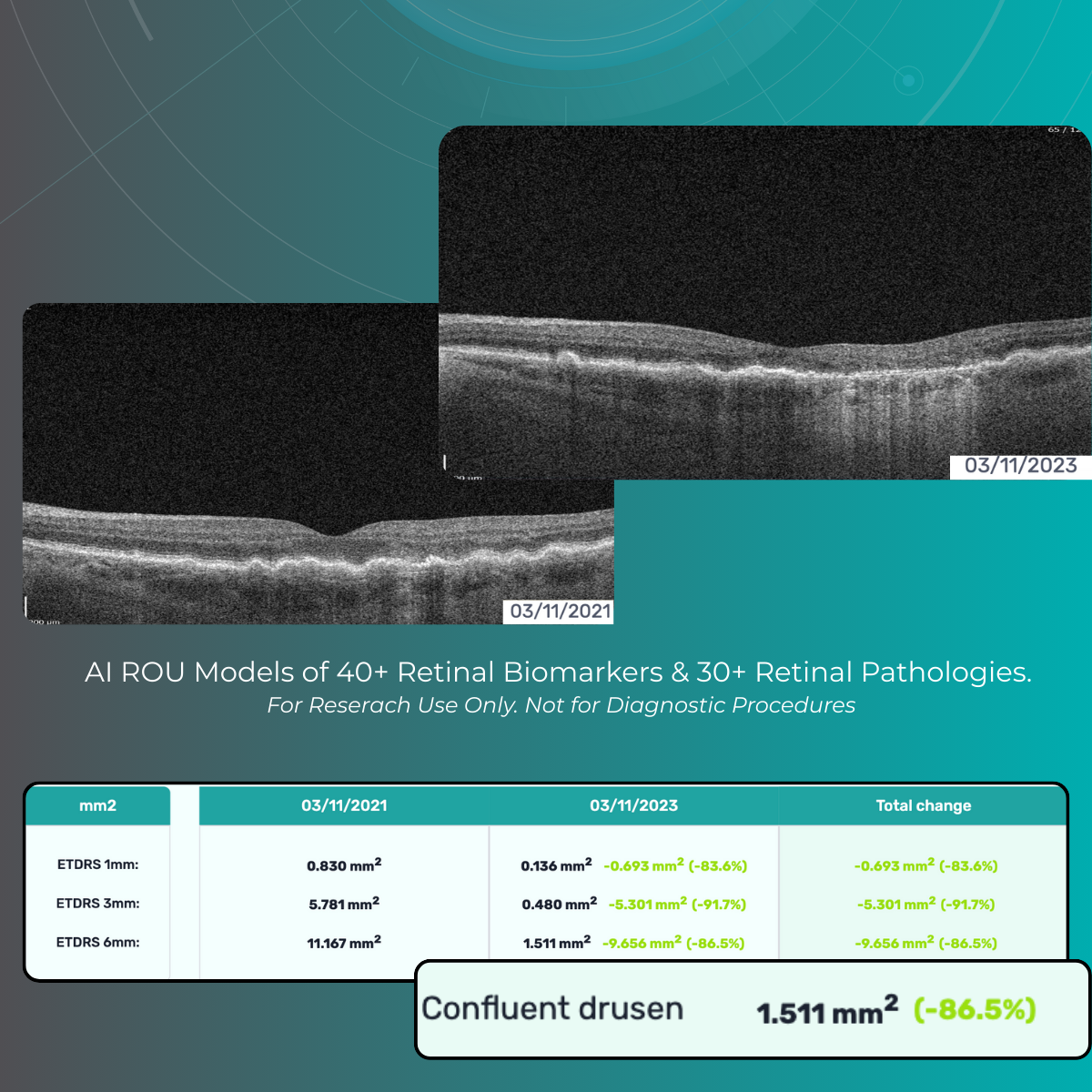
Clinical & Commercial benefits
We work with 9 OCT manufacturers to sync OCT data
Powering OCT research with AI models of 30+ retina pathologies & 40 + biomarkers
Working with historical OCT data using AI ROU Models
Supporting maintenance of patient care standards across all locations
Encourages systematic OCT image review to help avoid overlooked findings
Data-driven insights
Supporting consistent OCT image research across the workflow
Compatible with OCT devices from nine leading manufacturers
Displays maps, graphs, and metrics to support monitoring of retinal changes over time
Supporting patient understanding of retinal health through image-based information
Enable multiple locations to access and annotate OCT datasets simultaneously
- Centralized OCT Data Management
- Vendor-Neutral OCT Compatibility (9 manufacturers)
- Secure and Compliant Data Environment
- Seamless Workflow Integration
- Historical data analysis
- 40+ retinal biomarkers studied in research across 30+ retinal conditions. For Research Use Only. Not for diagnostic procedures
- Quantitative exploration of 40+ biomarkers for Research Use Only. Not for diagnostic procedures
- Secure Image Management System for OCT
- Vendor-Neutral OCT Compatibility (9 manufacturers)
- Seamless Workflow Integration
- Historical data analysis
- 40+ retinal biomarkers studied in research across 30+ retinal conditions. For Research Use Only. Not for diagnostic procedures
- Quantitative exploration of 40+ biomarkers for Research Use Only. Not for diagnostic procedures.
- Smart OCT data management





Medical Director