Altris AI for Dry AMD RUO Models for Research
Learn how Altris AI RUO Models support Dry AMD Research & Analysis
Book Intro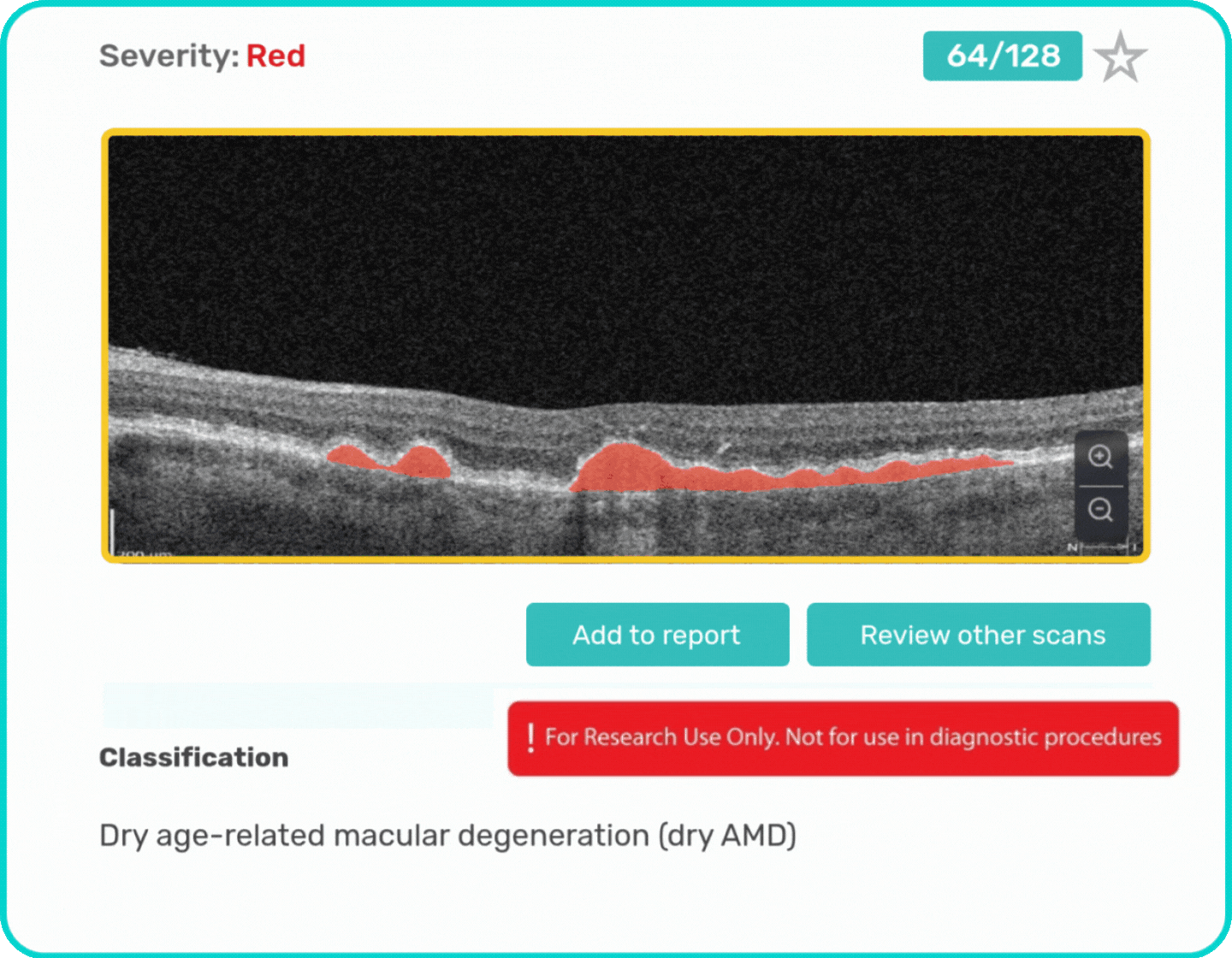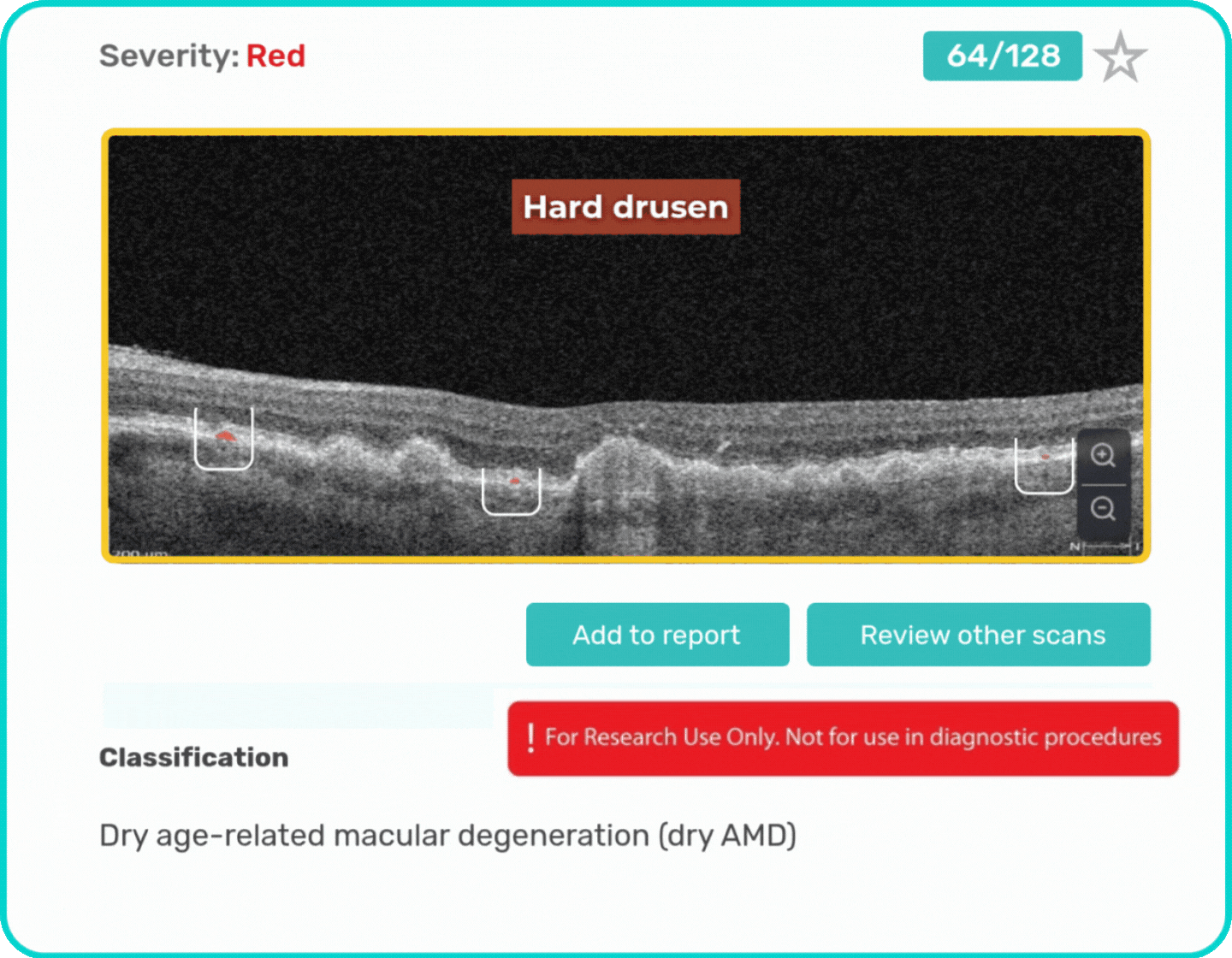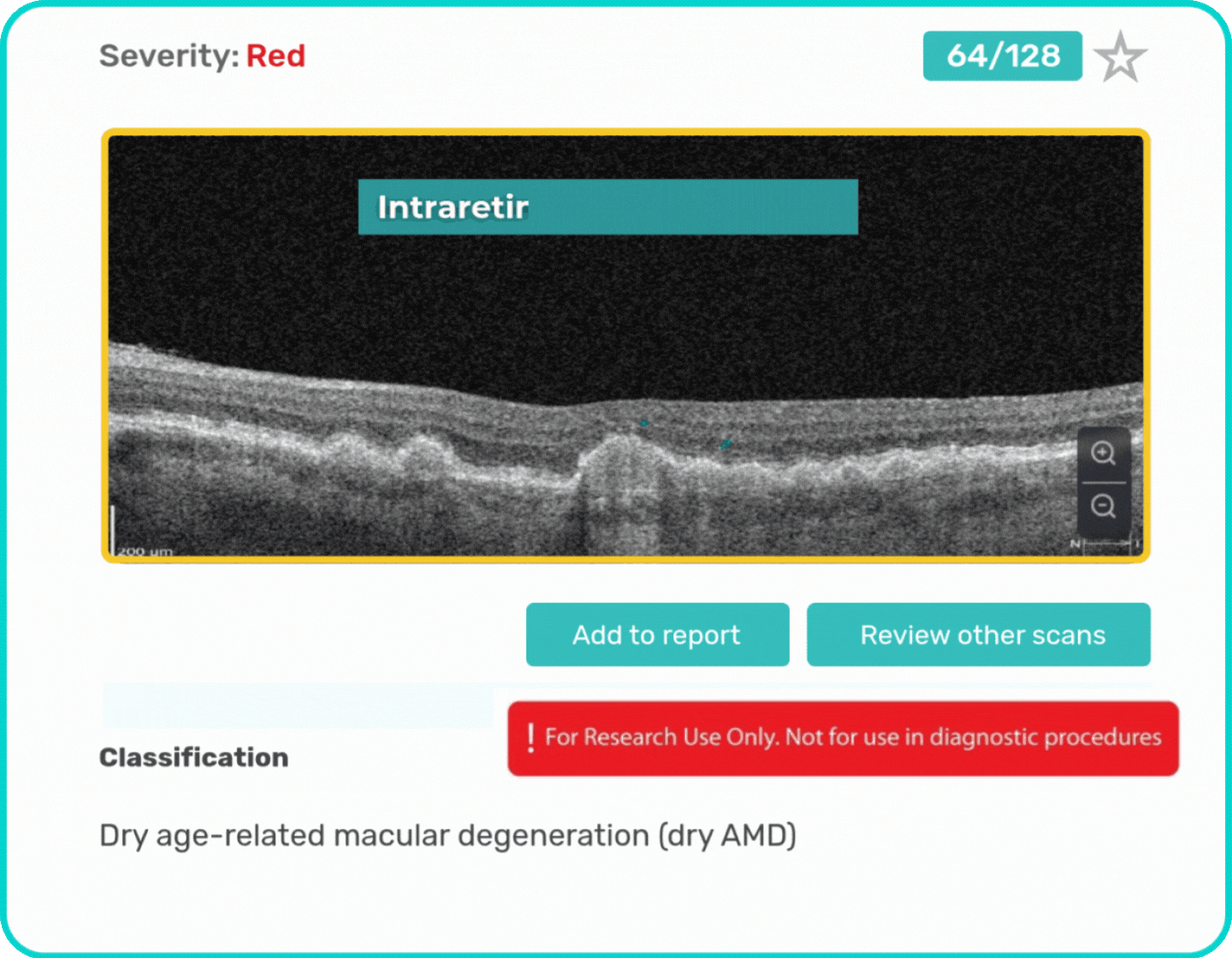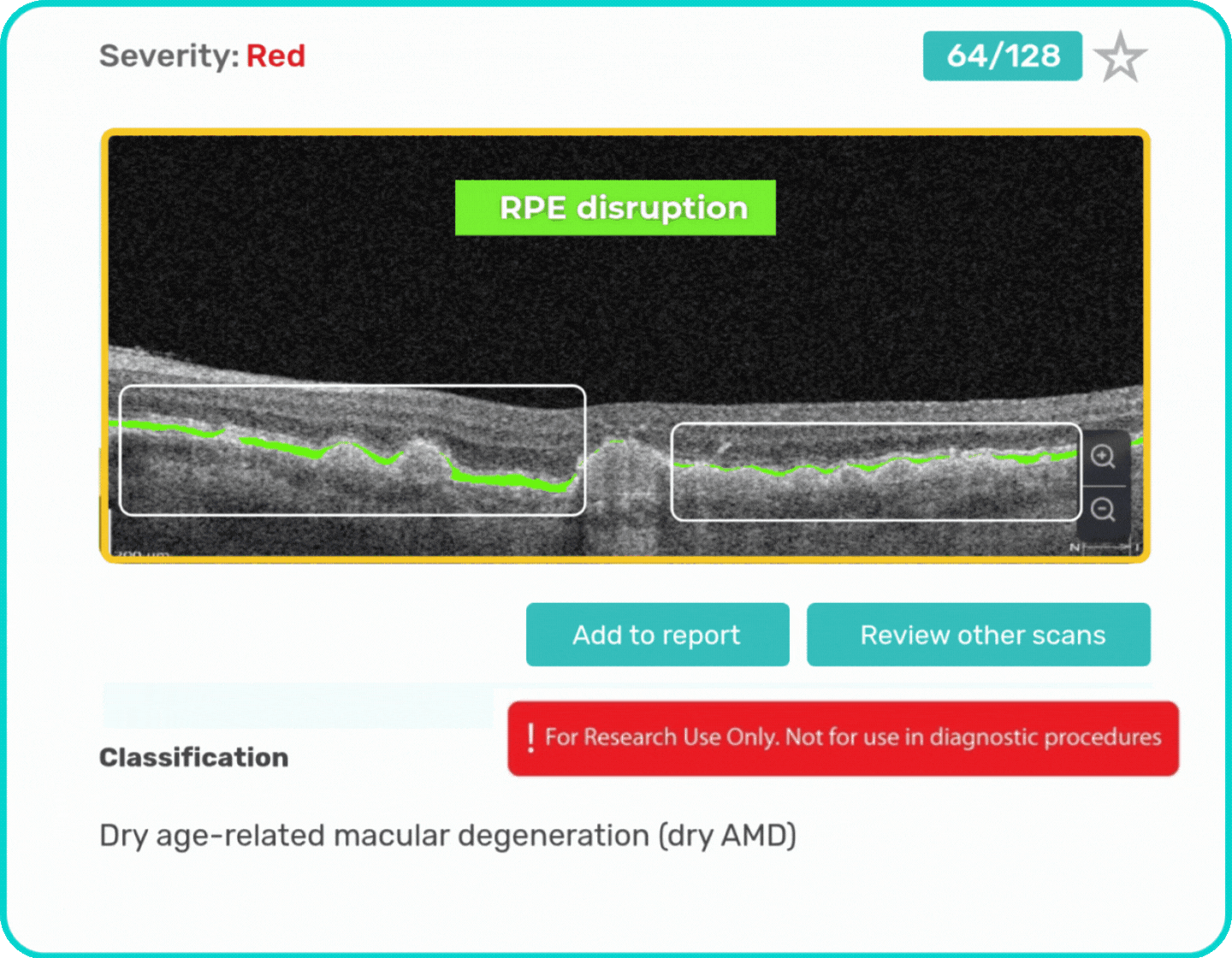
Altris IMS is an FDA-cleared (510(k)) image management system for OCT. Intended to support research workflows by enabling:
- Identification and visualization of biomarkers related to Dry AMD.
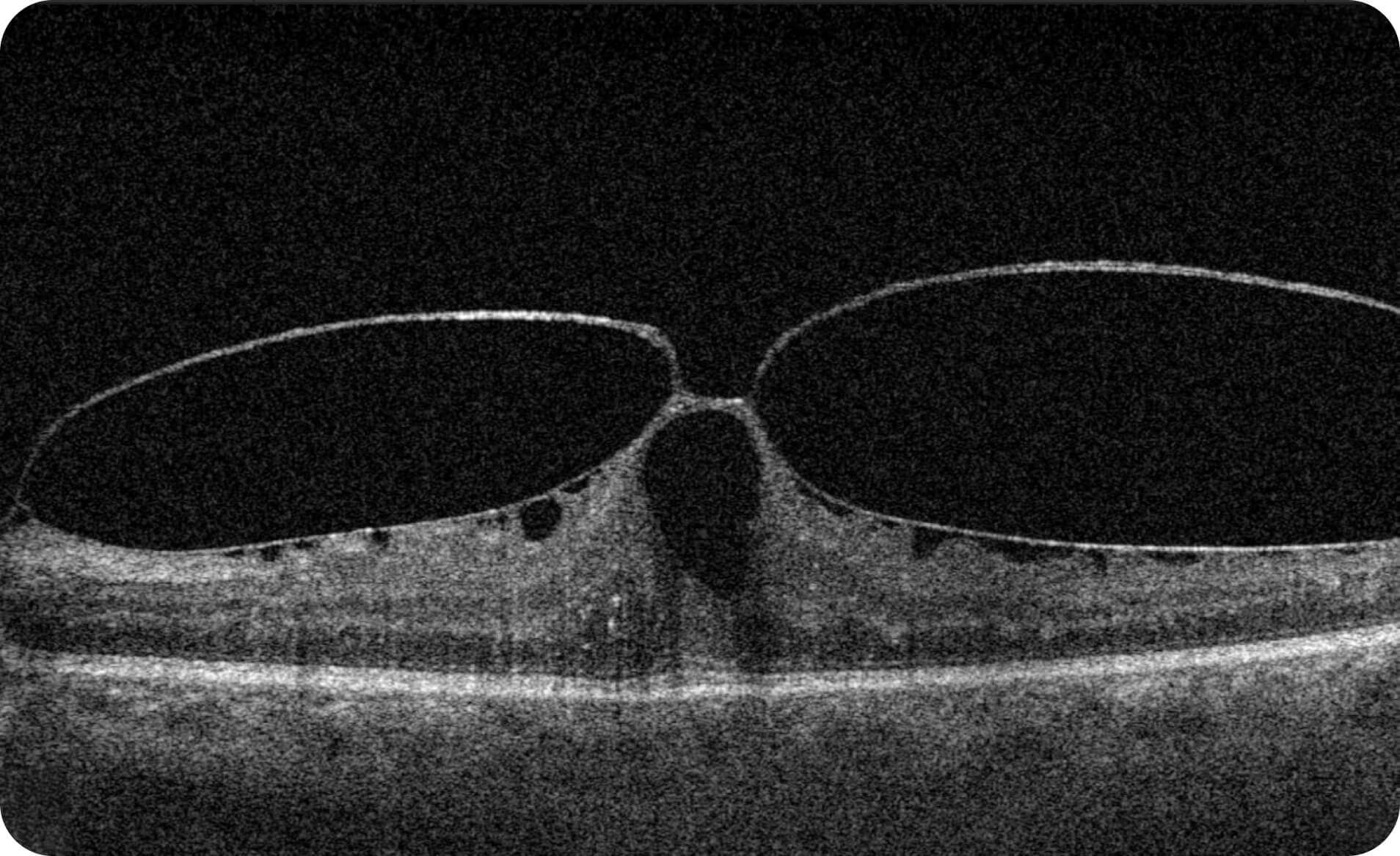
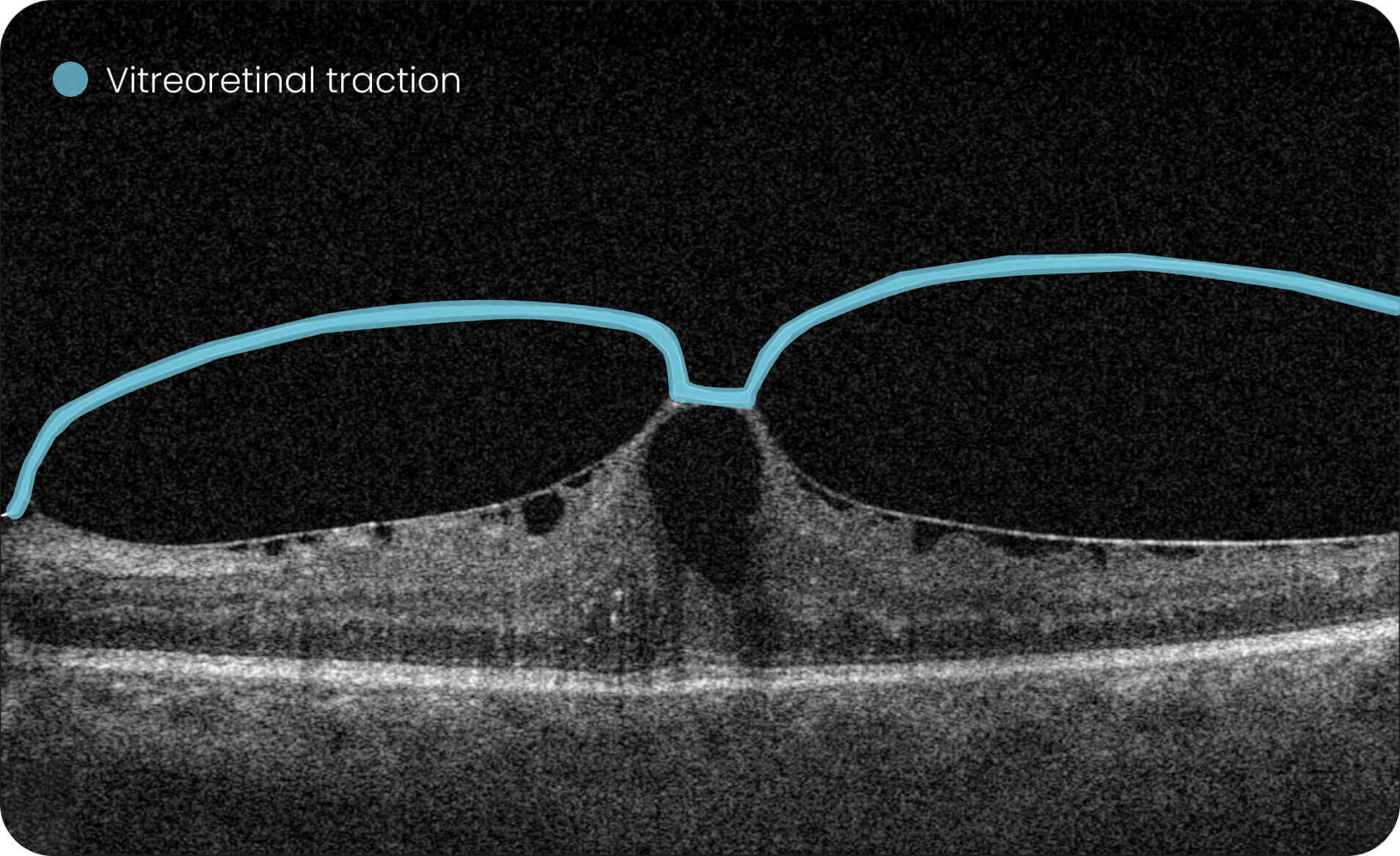
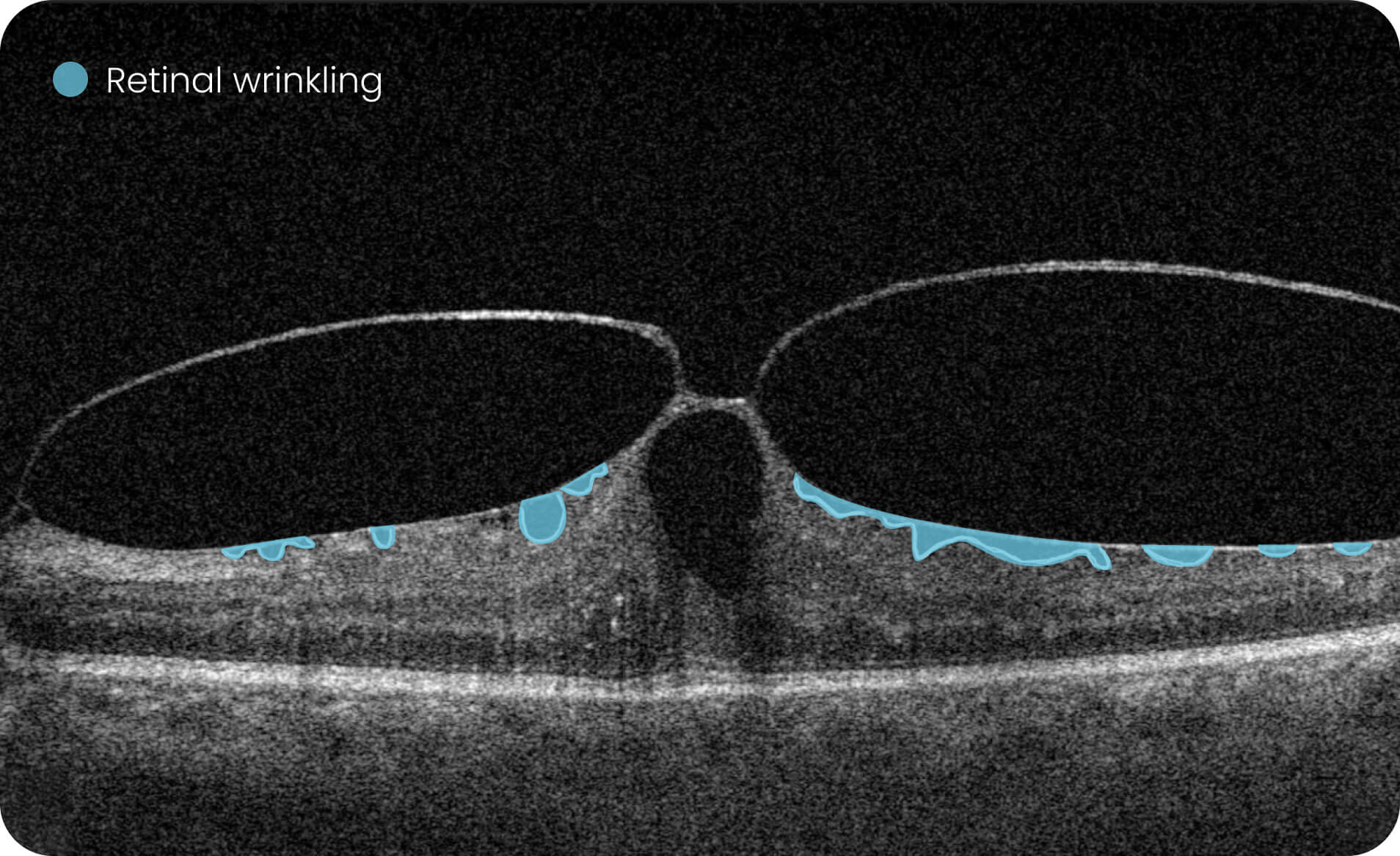
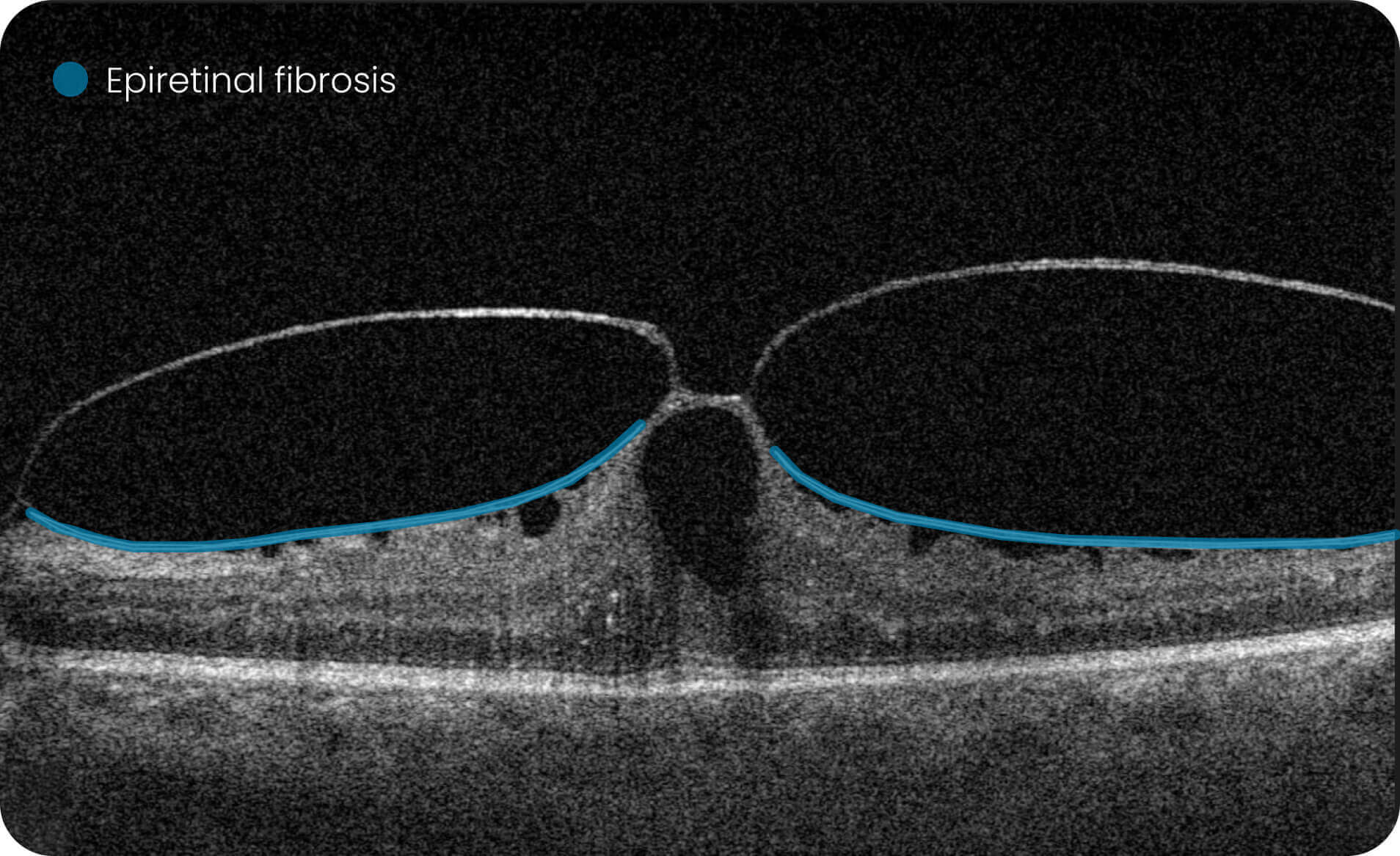
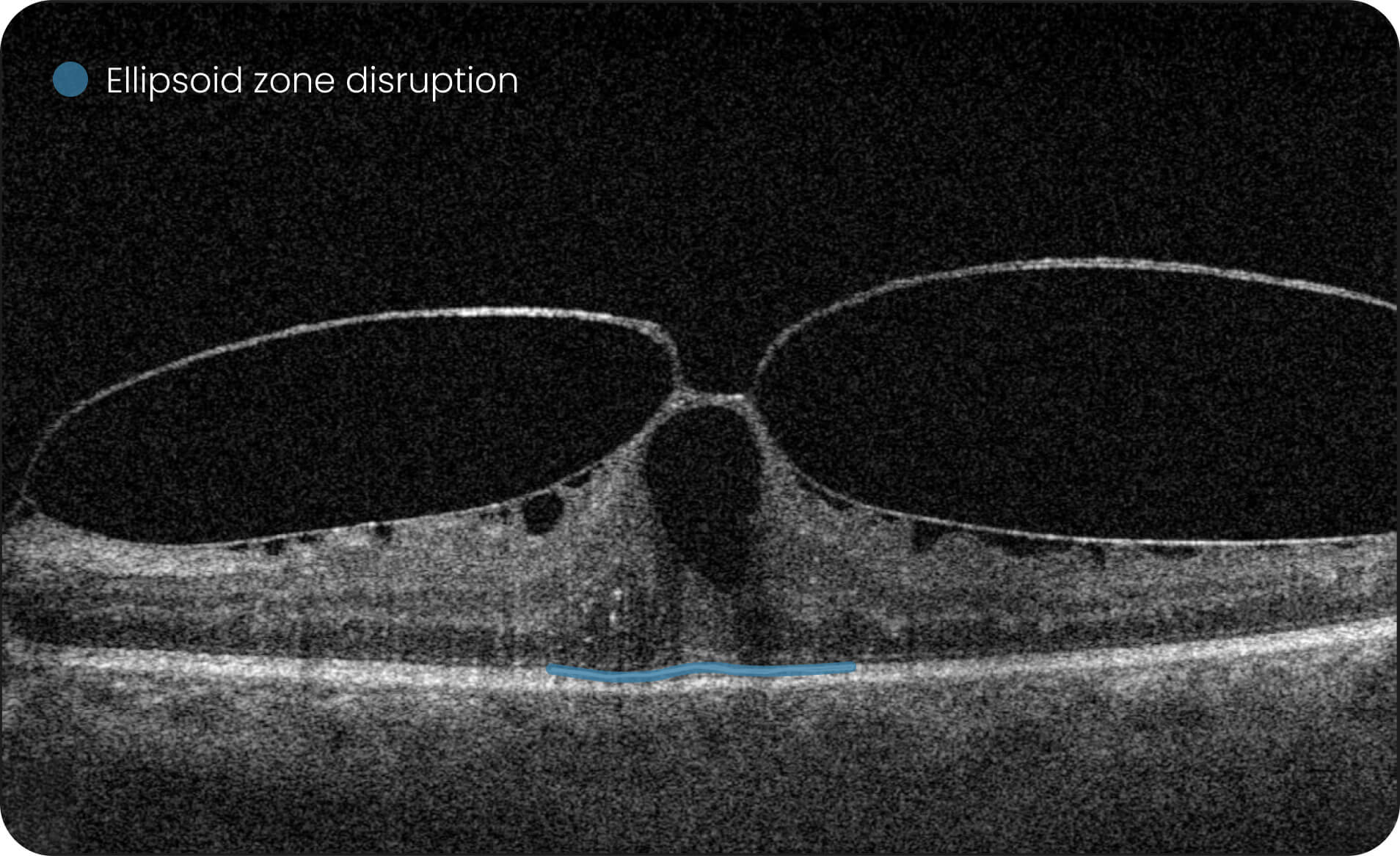
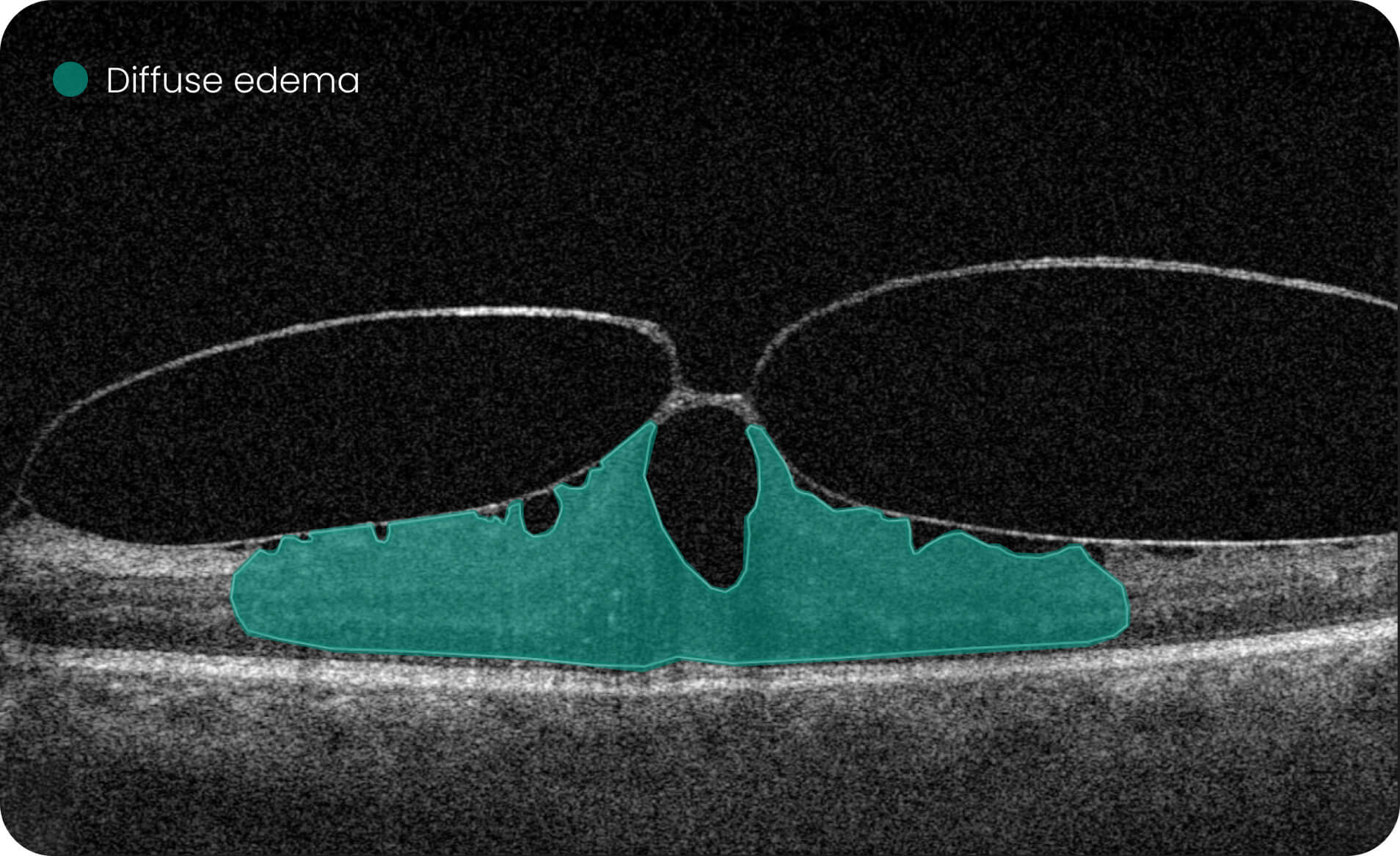
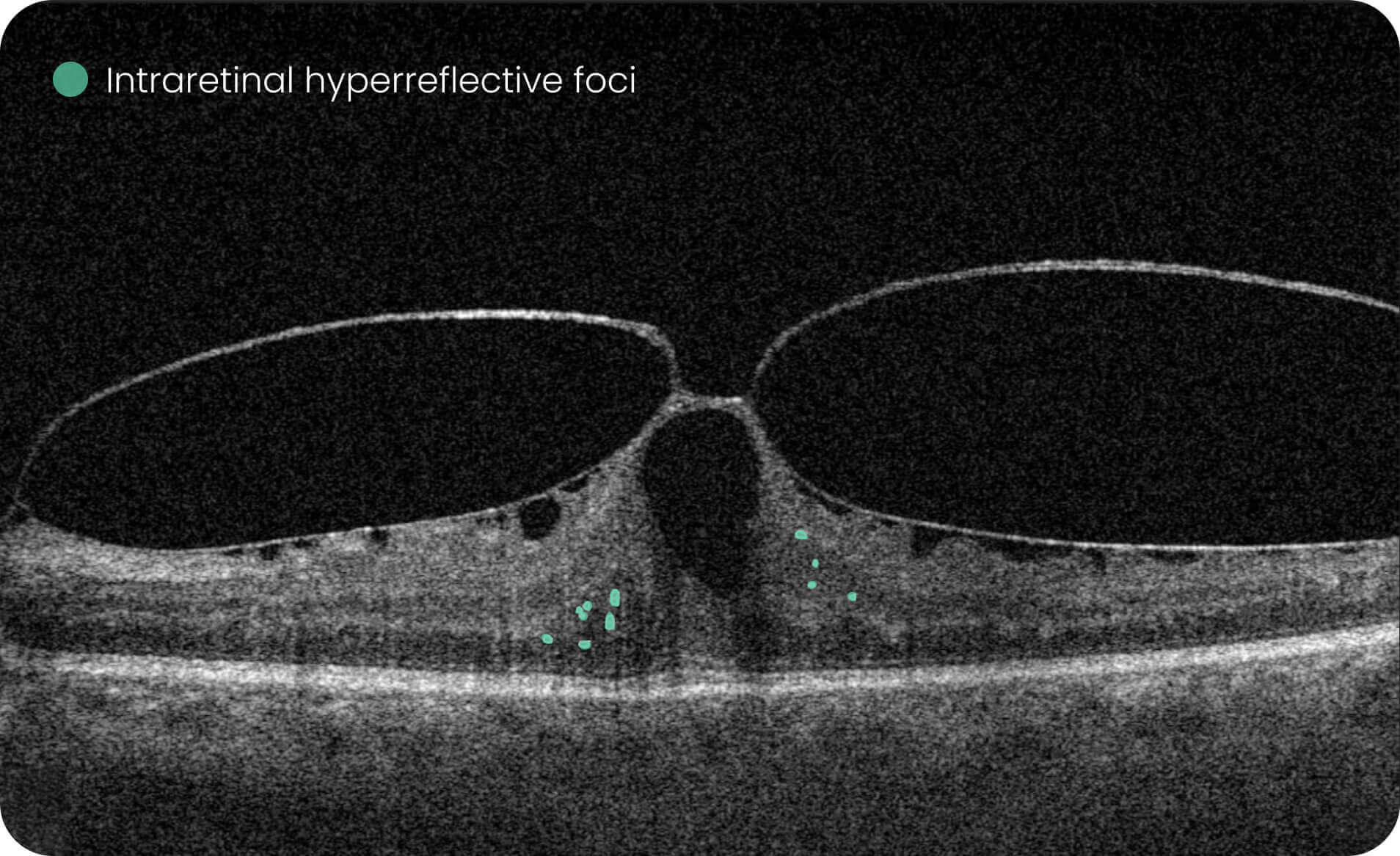
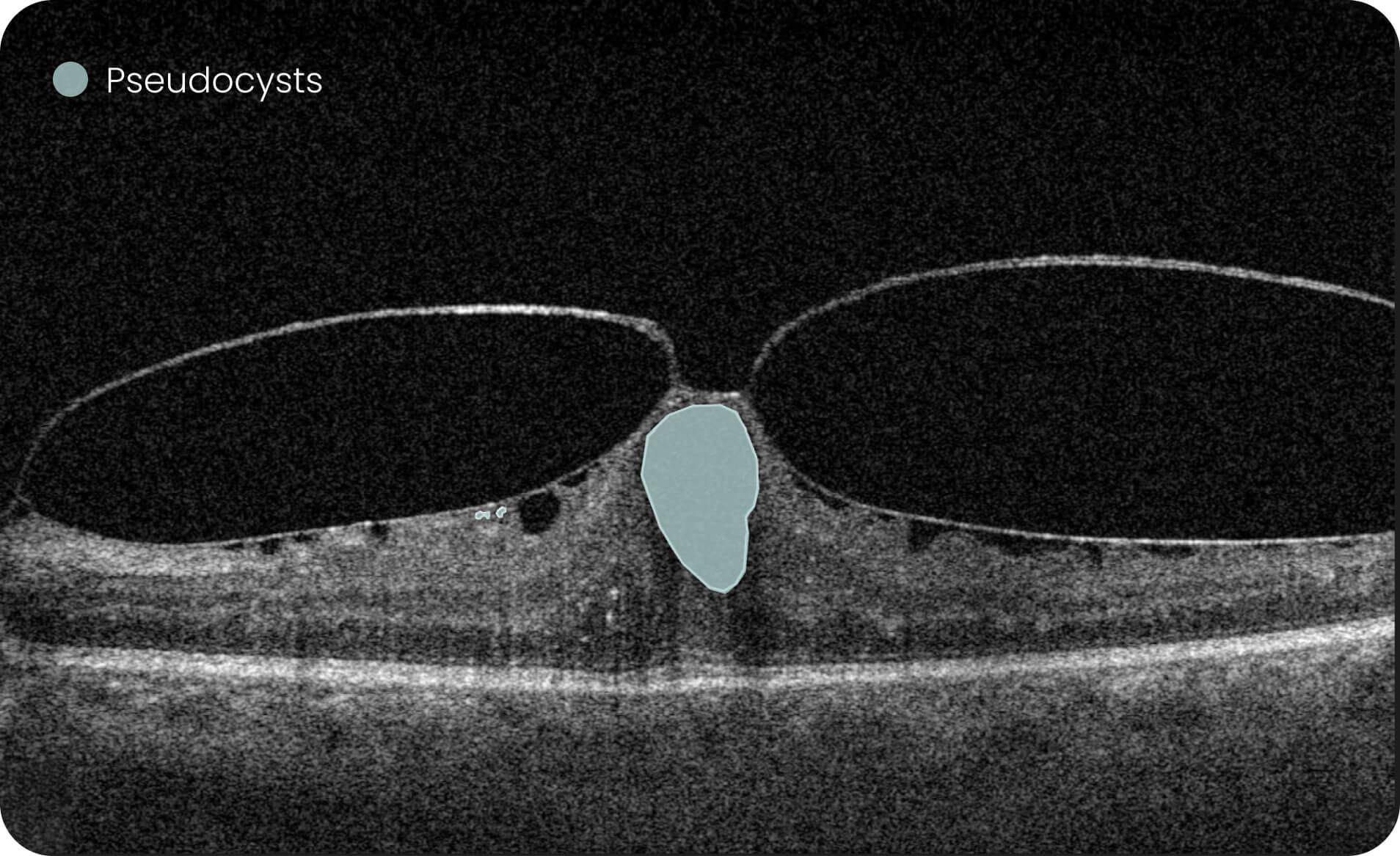
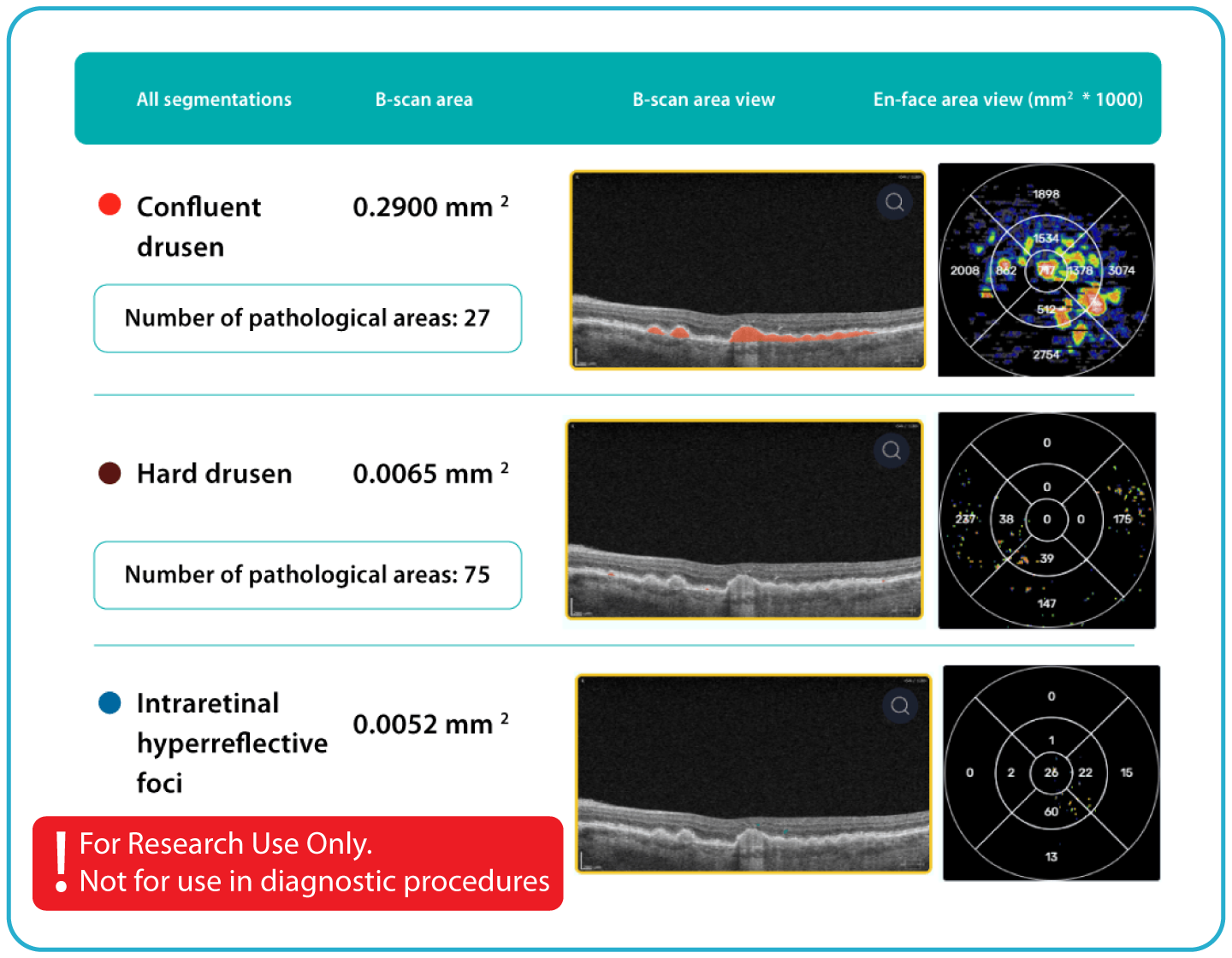
- Quantitative measurements of Dry AMD–related OCT imaging features, including confluent drusen, hard drusen, soft drusen, reticular pseudodrusen, RPE atrophy, hypertransmission, neurosensory retinal atrophy, vitelliform material, and chorioretinal scar.
- Automated comparisons of imaging data across multiple visits can be viewed through percentage-based displays, maps, and graphs.
Research Use Only. Not for diagnostic procedures.






Platform overview
Only practical features for eye care specialists and clinical research
The security of patients’ data is our top priority: we are GDPR compliant, all data is encrypted, CE-certified, and FDA- cleared (510k).
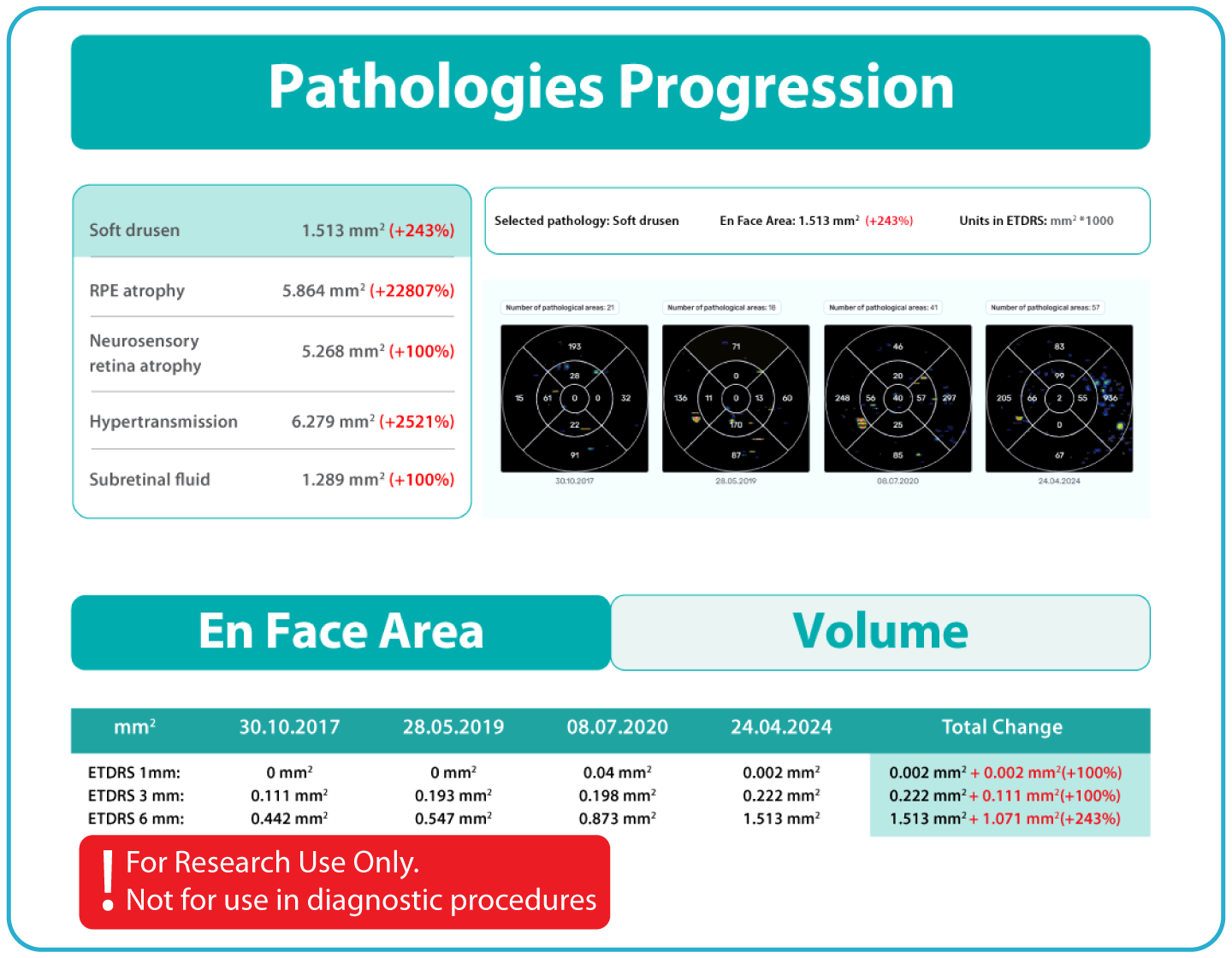
The system provides tools for research-focused review of longitudinal OCT data for US retina studies to observe changes associated with Dry AMD. Thus, the Pathology Progression module offers quantitative, image-derived measurements of selected Dry AMD-related features, including fine-scale changes, to support research analysis.
- Efficient exploration of imaging features associated with Dry AMD for research workflows.
- Quantitative characterization of biomarkers to support objective, repeatable research analysis.
- Longitudinal visualization tools to study changes over time in OCT-derived measurements.
- Research-focused review of drusen characteristics, including type, volume, area, and size, using the Drusen Count display.
- Reproducible, automated measurements enable structured evaluation of OCT imaging features for research and analytical purposes. Research Use Only. Not for diagnostic procedures.
How Altris IMS Works?
And how it supports the research and analysis of Dry AMD–related imaging features?
Altris IMS is a web-based platform developed with input from retina specialists to support eye-care professionals in reviewing and analyzing OCT imaging data.
Using a large repository of OCT scans with expert graphical annotations, the Altris AI Research Use Only (RUO) models are designed to:
AI components are for Research Use Only. Not for diagnostic or therapeutic purposes.
Formats
DICOM format will help you to extract maximum information. The system works with all data formats.
OCT equipment
Altris IMS tool is vendor-neutral. We work with all the OCT equipment producers
OCT reports
We create comprehensible OCT reports for patients and doctors
For Pharma
For innovative approach in pharma
Contact usWhat you get:
- Centralized OCT Data Management
- Historical Data Analysis
- Vendor-neutral analysis of OCT scans (8 manufacturers)
- Data Security & Compliance
- Additional capabilities for research
- 40+retinal biomarkers studied in research across 30+ retinal conditions. For Research Use Only. Not for diagnostic procedures.
- Quantitative exploration of 40+biomarkers for Research Use Only. Not for diagnostic procedures.
- Reports
For Eye Care
IMS for Ophthalmology and Optometry
Contact us:What you get:
- Centralized OCT Data Management
- Vendor-Neutral OCT Compatibility (8 manufacturers)
- Historical OCT Data Analysis
- Seamless Clinical Workflow Integration
- Informative OCT reports
- 40+retinal biomarkers studied in research across 30+ retinal conditions. For Research Use Only. Not for diagnostic procedures.
- Quantitative exploration of 40+biomarkers for Research Use Only. Not for diagnostic procedures.