Diabetic retinopathy screening and treatment: a complete guide
Table of Contents
- What are the diabetic retinopathy screening methods?
- Fundus images in DR screening
- Can OCT detect diabetic retinopathy?
- What does diabetic retinopathy look like on OCT?
- What is optimal diabetic retinopathy screening frequency?
- What is the best treatment for diabetic retinopathy?
- Diabetic retinopathy management: key takeaways
Diabetic retinopathy (DR) remains the leading cause of irreversible vision loss among working-age adults worldwide. According to the International Diabetes Federation (IDF), one in three patients with diabetes shows signs of DR, and 10% develop diabetic macular edema (DME). Early diagnosis, systematic screening, and individualized monitoring are essential to prevent vision loss.
What are the diabetic retinopathy screening methods?
Modern methods of DR screening include:
- Telemedicine platforms – enable automated transmission of fundus images
- Mobile fundus cameras – Wi-Fi–enabled devices for field examinations
- Smartphone-based platforms – use specialized lenses for retinal imaging
- Optical coherence tomography (OCT) – used to detect early retinal changes and diabetic macular edema, complementing fundus photography
- AI-based systems – solutions for automated image analysis for fundus and OCT
In practice, these methods are often combined. For example, patients may undergo fundus photography, after which images are sent to telemedicine centres and analysed by AI algorithms. More complex cases are then referred to ophthalmologists.
DR screening is frequently incorporated into annual diabetes check-ups conducted by primary care physicians trained in basic fundus photography. This approach, already successfully implemented in several EU countries, has reduced the incidence of severe DR.
Innovations in DR screening have broadened access for rural residents, older adults, and individuals with limited mobility. Integration into national e-health systems enables automated reminders and electronic medical record linkage, incorporating laboratory data (HbA1c, blood pressure) alongside retinal images.
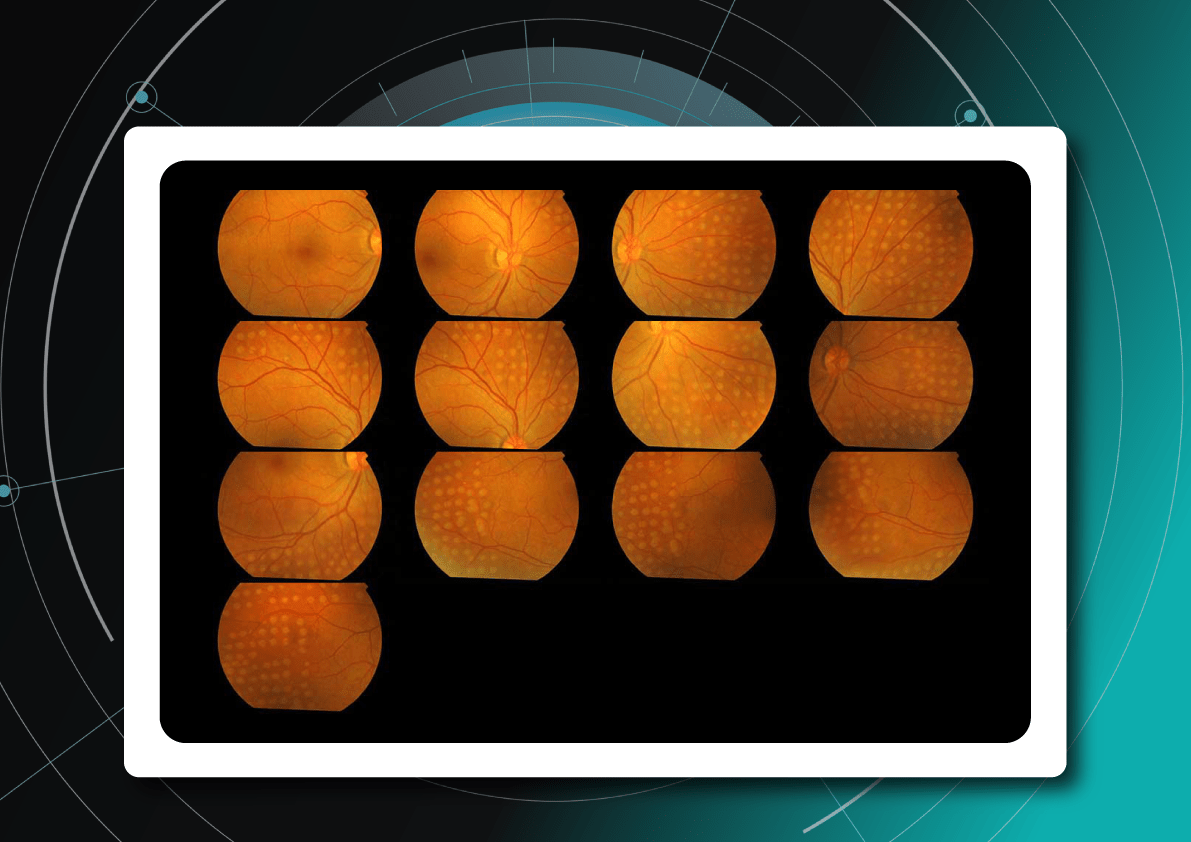
Fundus images in DR screening
Fundus photography is the optimal primary screening method due to its high diagnostic yield, cost-efficiency, simplicity, and ability to integrate with AI and telemedicine solutions.
It enables detection of microaneurysms, hemorrhages, exudates, and neovascularization, often before symptoms arise. National screening programs rely heavily on digital fundus imaging, which, when combined with AI, provides an efficient platform for mass DR detection.
Advances in fundus imaging for diabetic retinopathy have improved efficiency. Modern non-mydriatic cameras deliver high-quality images without pupil dilation, while automated image analysis supports rapid identification of suspicious cases. Cloud storage and telemedicine platforms facilitate remote evaluation, increasing coverage in regions with limited ophthalmology services.
Next-generation wide-field cameras further enhance detection by capturing peripheral pathology. Some devices also generate automated annotations, reporting lesion type, DR stage, and DME presence, thereby standardizing interpretation and expediting clinical decision-making.
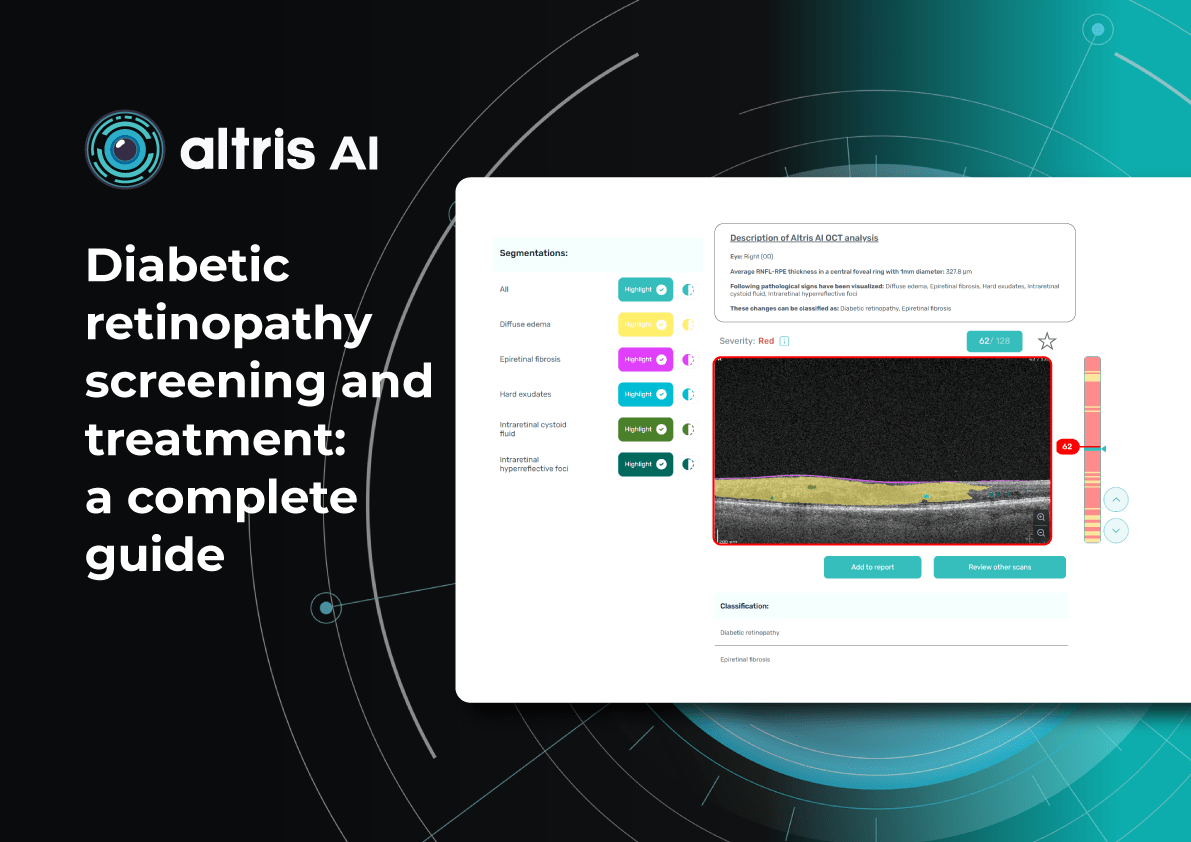
Can OCT detect diabetic retinopathy?
Yes. OCT can detect early structural changes in the retina and is increasingly used to complement standard diabetic retinopathy screening.
- Role in DR screening – While not a primary screening tool, OCT is now widely applied alongside fundus photography. It is especially valuable for detecting early diabetic macular edema (DME) and subtle morphological changes in the central retina not visible during ophthalmoscopy.
- High-resolution imaging – OCT visualizes changes such as photoreceptor layer disruption, subclinical intraretinal fluid, neurosensory retinal thickening, and foveal edema. These findings often appear before clinically significant macular edema.
- Differential diagnosis – OCT also helps identify other causes of vision loss in diabetic patients, for example, ruling out age-related macular degeneration.
- Clinical evidence – Studies confirm that combining OCT with fundus photography increases diagnostic accuracy for DME. Experts therefore recommend this approach for patients with long-standing diabetes, poor glycemic control, or vision complaints.
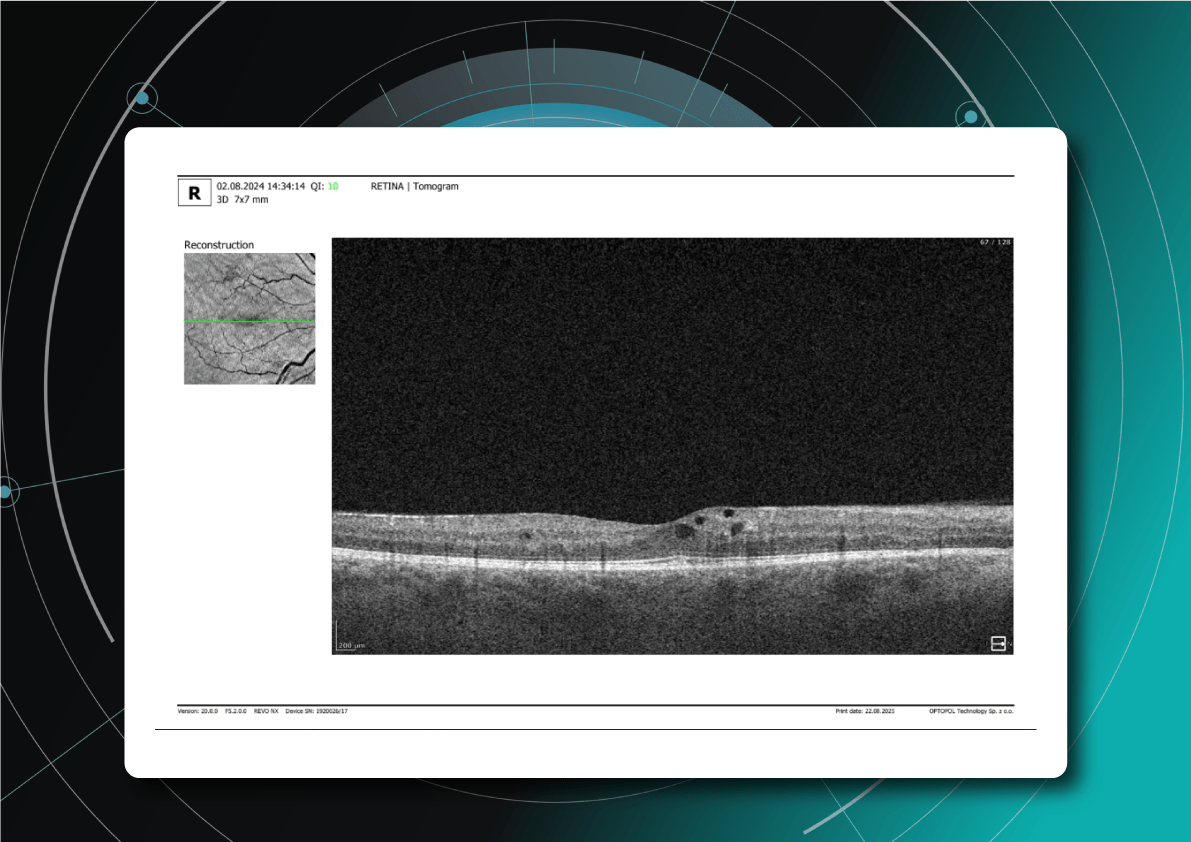
What does diabetic retinopathy look like on OCT?
On OCT, diabetic retinopathy (DR) can appear as a combination of retinal structural damage, fluid accumulation, and microvascular changes that may not be visible on fundus photography.
Typical OCT findings in DR include:
- Photoreceptor damage – loss of outer retinal layers, especially the ellipsoid zone
- Intraretinal hyperreflective foci, hard exudates
- Microaneurysms – visible as small, round changes within the retina
- Retinal thickness changes and neuroepithelial layer atrophy
- Diabetic macular edema – with intraretinal hyporeflective cystoid spaces and neuroepithelial swelling
- Subretinal fluid – resulting from increased vascular permeability
- DRIL – disorganization of inner retinal layers, associated with poor prognosis
- Epiretinal membranes – potential precursors to retinal detachment
Advanced findings
OCT can also reveal proliferative changes and tractional zones, which may progress to tractional retinal detachment.
OCTA insights
Beyond structural analysis, OCT angiography (OCTA) allows visualization of retinal microvascular changes without the contrast injection. OCTA helps identify areas of neovascularization, capillary network disruption, and the degree of macular ischemia.
What is optimal diabetic retinopathy screening frequency?
The screening frequency for diabetic retinopathy is tailored to diabetes type, disease stage, and risk factors:
Type 1 diabetes
- First screening: 3–5 years after diagnosis (due to onset in children and young adults)
- Then annually, if no DR is detected
- If DR is present, frequency depends on severity
Type 2 diabetes
- Screening at diagnosis, as DR may already be present.
- If no DR, repeat every 1–2 years.
Patients with confirmed DR
- No visible DR, mild non-proliferative diabetic retinopathy (NPDR), no DME — every 1–2 years
- Moderate NPDR — every 6–12 months.
- Severe NPDR — every 3 months.
- Proliferative DR (PDR) — monthly, with regular OCT monitoring of the macula.
- DME — monthly if center-involving, every 3 months if not.
Pregnant women with type 1 or type 2 diabetes
- Screening before conception or in the first trimester, with follow-up each trimester and postpartum
- Screening is not required for gestational diabetes without pre-existing diabetes
Post-treatment patients (laser or vitrectomy)
- Typically, every 3–6 months during the first year, individualized based on retinal stability
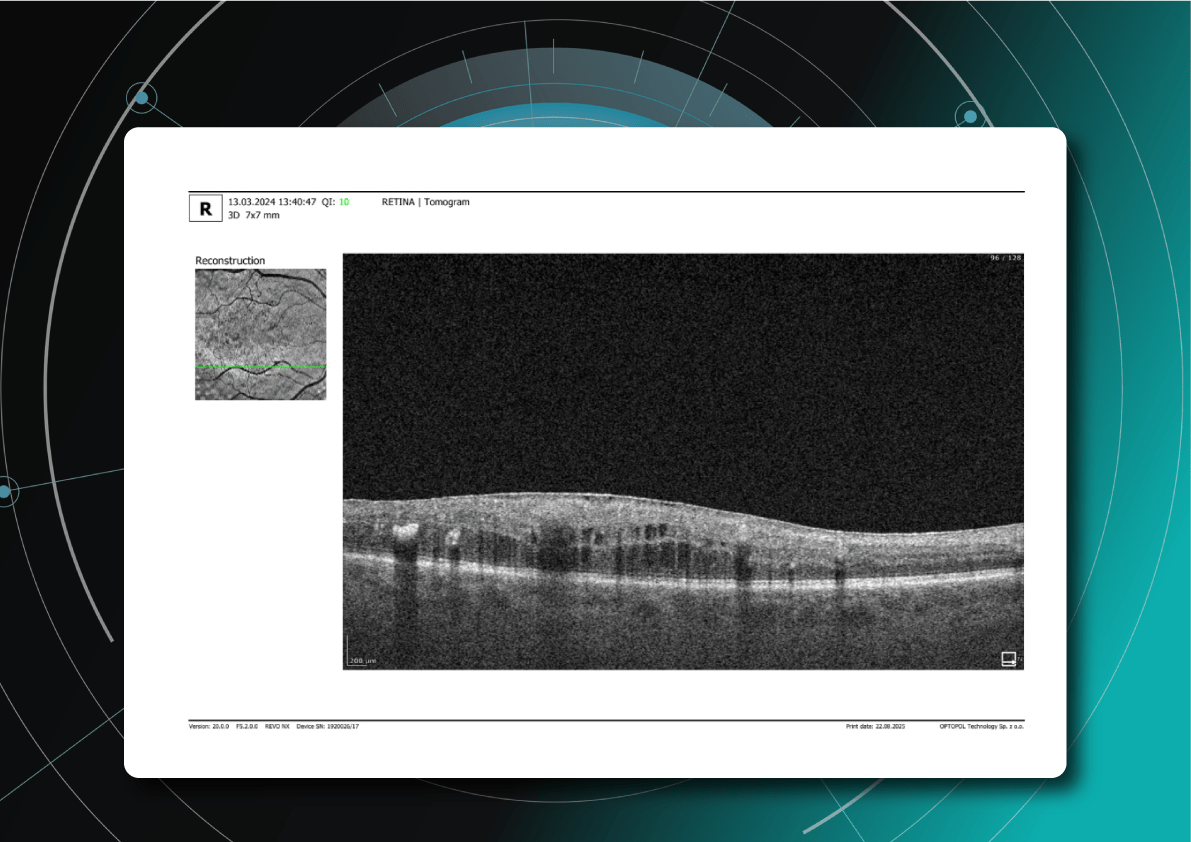
Monitoring of diabetic retinopathy progression
Ongoing diabetic retinopathy monitoring is essential to detect early signs of progression and guide treatment decisions. A key focus in monitoring is diabetic macular edema (DME), which represents fluid accumulation in the macula due to leakage from damaged retinal vessels. DME is a common complication of DR and the leading cause of vision loss in diabetic patients. OCT plays a central role in detecting DME and identifying structural changes that indicate disease progression.
OCT biomarkers in DME
OCT enables precise visualization of retinal layers with micron resolution, confirming DME presence and providing prognostic biomarkers for treatment selection and monitoring.
The main OCT biomarkers in DME include:
- Cystoid hyporeflective intraretinal spaces – usually in the inner nuclear layer (INL) or outer plexiform layer (OPL). Their number, size, and location correlate with edema severity. Large or confluent spaces may indicate chronicity and a worse prognosis.
- Subretinal fluid – accumulation between the neurosensory retina and retinal pigment epithelium. Often associated with a better visual prognosis, but requires close monitoring and consideration in anti-VEGF therapy.
- Central macular thickening – a key marker of treatment effectiveness and disease activity.
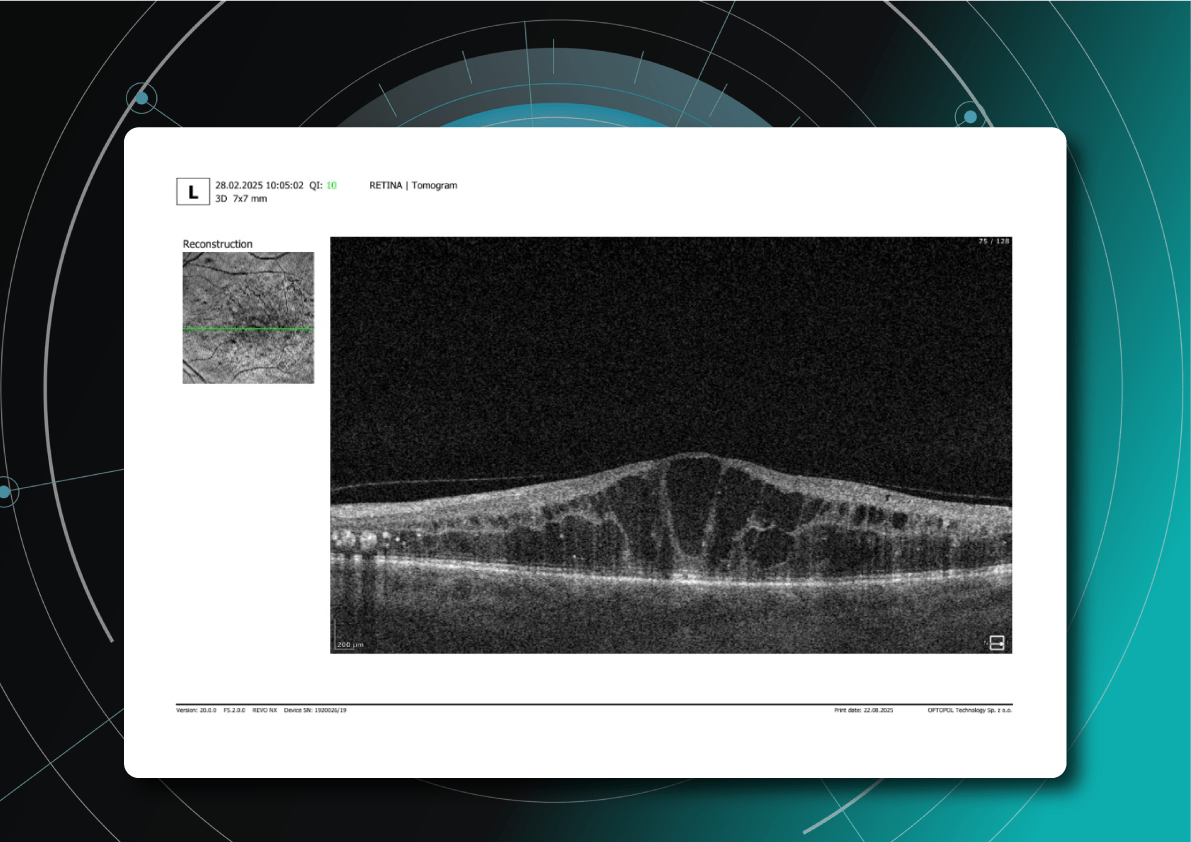
OCT red flags in DR progression
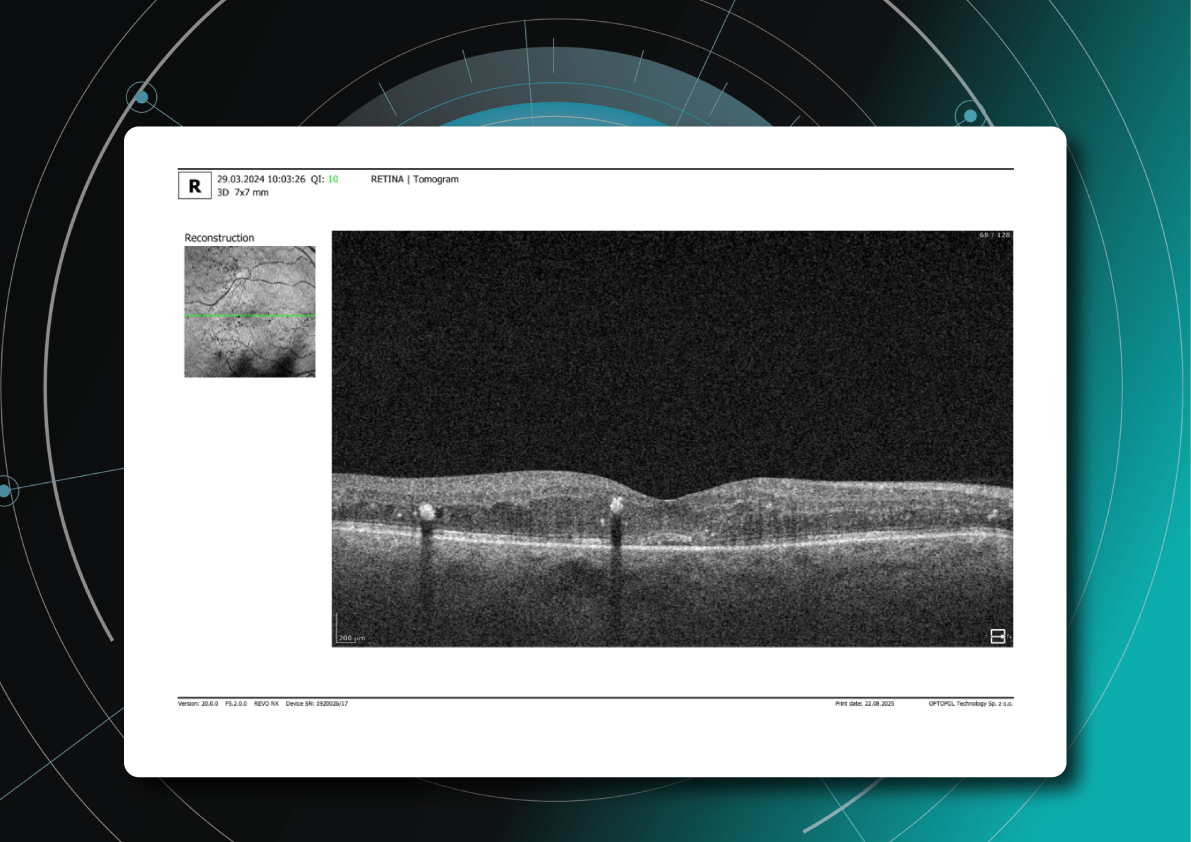
Beyond DME, OCT helps identify broader signs of DR worsening that require therapy reassessment:
- Progressive central macular thickening despite treatment
- Increase in intraretinal or subretinal fluid, or enlargement of cystoid spaces
- New hyperreflective foci, reflecting inflammatory activity (these may precede hard exudates or RPE changes)
- Development or progression of disorganization of inner retinal layers (DRIL), an independent predictor of poor prognosis, even when orphological improvement is seen on OCT
- Ellipsoid zone disruption, indicating photoreceptor damage
- Signs of macular ischemia, although better evaluated with OCTA, indirect signs on OCT may include thinning of the inner retinal layers.
- Tractional changes, such as epiretinal membranes, inner retinal stretching, or macular traction
The appearance of these OCT features should prompt clinicians to reconsider therapy, whether by switching anti-VEGF agents, introducing steroids, using combination therapy, or referring patients for surgical evaluation when traction is present.
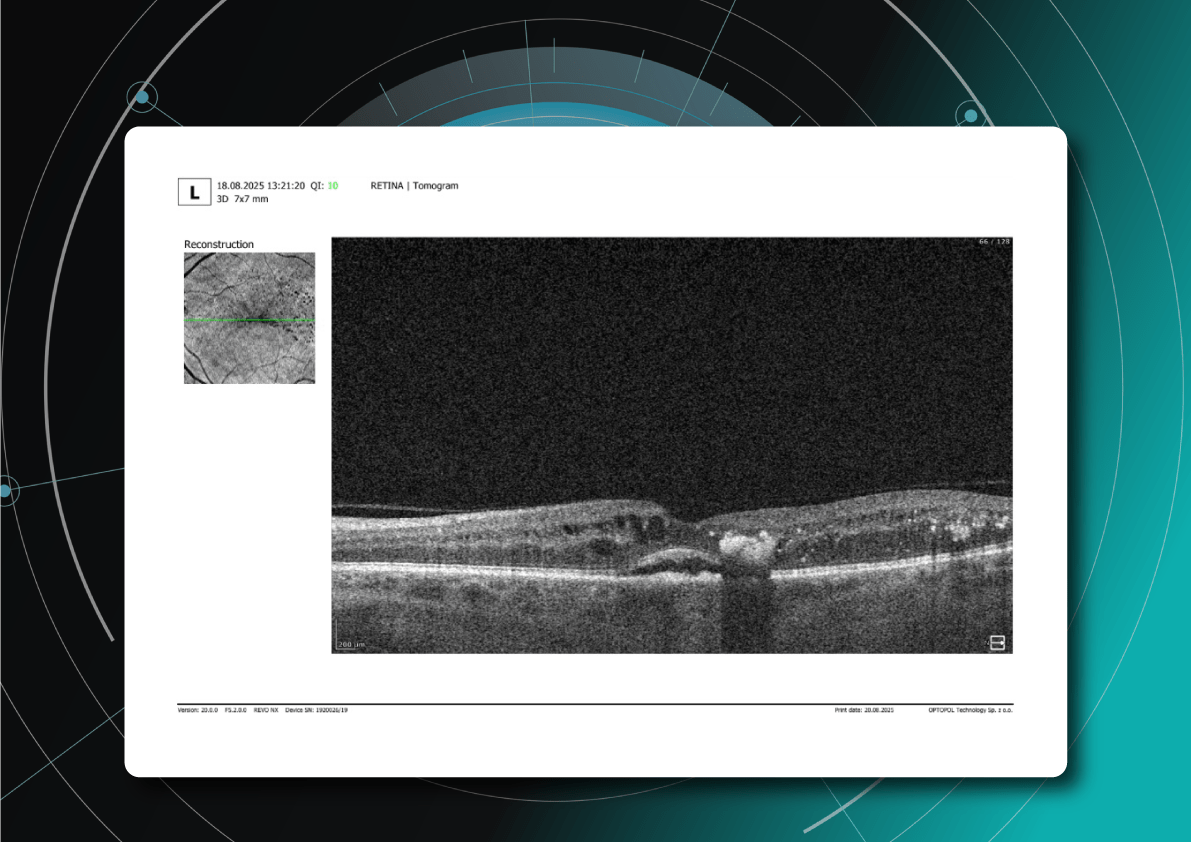
What is the best treatment for diabetic retinopathy?
The treatment of diabetic retinopathy is based on a comprehensive approach that takes into account not only the disease stage, but also individual patient characteristics, OCT findings, comorbidities, and prognostic biomarkers. Modern strategies combine preventive, pharmacological, and surgical methods, as well as personalized medicine tools based on retinal imaging.
Criteria for treatment selection
The choice of therapy is guided by the following parameters:
- DR stage – non-proliferative, proliferative, with or without DME
- Form of macular edema – focal, diffuse, with or without subretinal fluid
- Presence of DRIL, EZ disruption, ischemic changes on OCTA
- Response to previous treatment – anti-VEGF, steroids, laser
- Comorbidities – renal insufficiency, hypertension, poor adherence
For low-risk patients, observation or focal laser may be sufficient. Patients with significant DME usually require anti-VEGF or steroid injections. Those with proliferative DR often undergo panretinal laser photocoagulation or vitrectomy.
Diabetic retinopathy treatment methods
The main treatment options for diabetic retinopathy include pharmacotherapy, laser therapy, surgical intervention, and personalized approaches based on OCT.
1. Pharmacotherapy: anti-VEGF and steroids
Anti-VEGF agents such as aflibercept, ranibizumab, and bevacizumab are the first-line therapy for diabetic macular edema. They are especially effective in patients with pronounced edema and without ischemia.
New drugs with extended duration of effect, including port delivery systems, are becoming available.
Steroids are used when DME is persistent, when patients do not respond to anti-VEGF therapy, or in cases with an inflammatory phenotype.
2. Laser therapy
Injections have largely replaced laser therapy in the treatment of DME. However, panretinal photocoagulation remains the standard treatment for proliferative DR.
Subthreshold micropulse laser is increasingly applied for focal edema, as it minimizes tissue damage.
3. Surgical treatment
Vitrectomy is recommended in cases of tractional macular edema, vitreous hemorrhage, or retinal detachment.
4. Personalization with OCT
Modern treatment protocols use OCT biomarkers to tailor therapy and improve prognosis.
Patient education and multidisciplinary care
DR treatment outcomes strongly depend on adherence. Patients must be informed about the need for regular injections, monitoring, and systemic control. Coordinated care involving ophthalmologists, endocrinologists, and family doctors helps maintain stable glycemic control and slows DR progression.
Diabetic retinopathy management: key takeaways
Diabetic retinopathy is a progressive disease, but modern diagnostics and treatments make it possible to preserve vision and improve outcomes. OCT and OCTA have become essential tools for early detection, risk assessment, and personalized therapy planning. Effective management combines pharmacotherapy, laser treatment, surgery, and patient education. Multidisciplinary care and strong patient adherence remain crucial for long-term success. With timely monitoring and tailored treatment, the progression of diabetic retinopathy can be significantly slowed.
Disclaimer: USA FDA 510(k) Class II; Altris Image Management System (Altris IMS); AI/ML models and components intended to use for research purposes only, not for clinical diagnosis purposes.
